Information
-
Document No.
-
Select Site
-
Building Number, Room Number/Suite
-
Conducted on
-
The audit conducted by:
-
Personnel Present:
Introduction & Instruction
-
Scope information
-
To complete this audit accurately, non-compliance shall be supported by evidence, including photographs, specific locations, and detailed notes that describe the findings. Notes should be used to detail statements from auditees or persons present at the physical inspection. Evidence should also be included for demonstrating compliance where relevant. Hazards identified that present a Critical Risk to the Health and Safety of the persons shall be immediately reported via GSafe Incident Reporting Module and work should cease until the hazard is controlled.
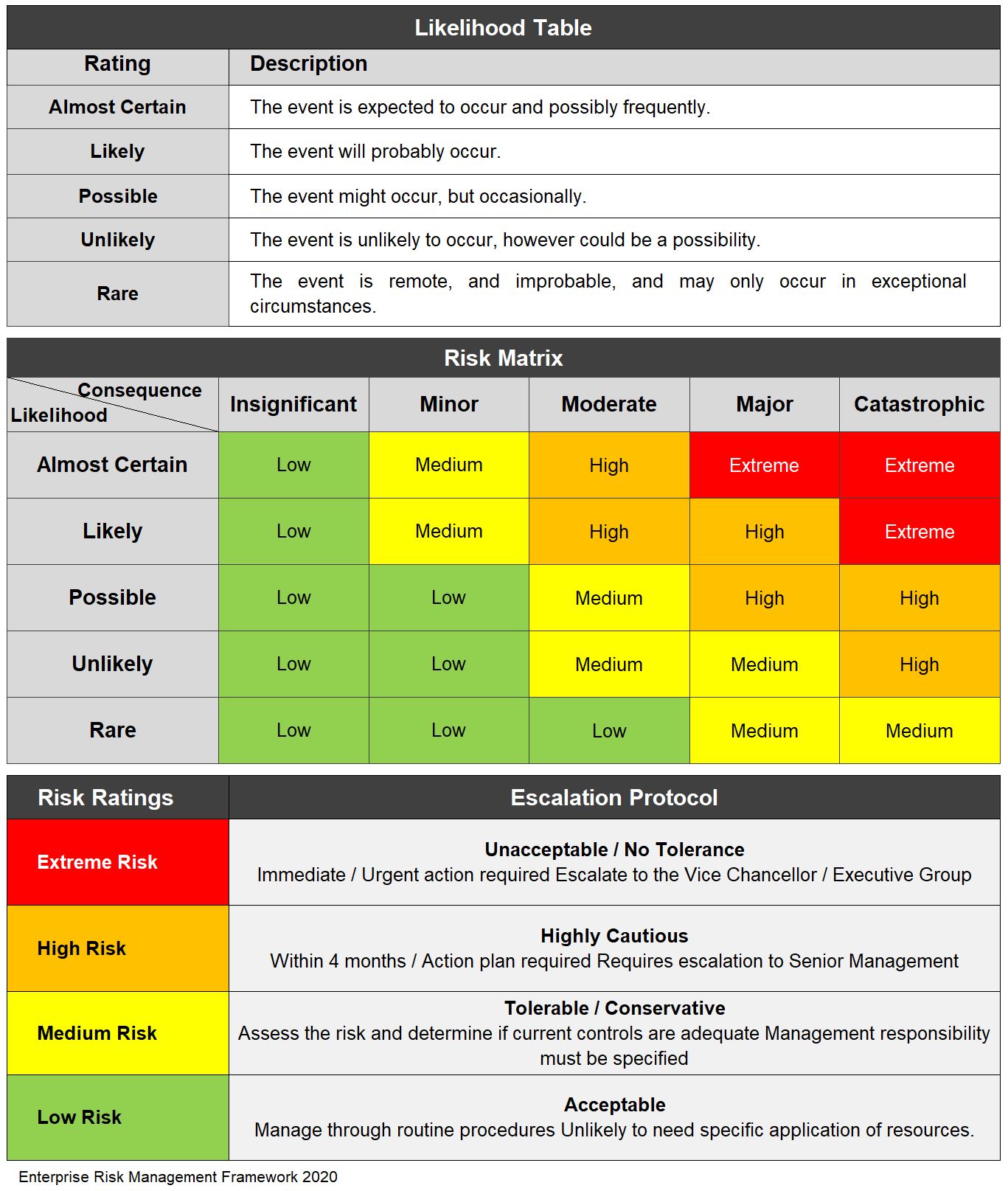
When assessing risks in the audit process, please refer to the Risk Matrix given below , giving consideration to the Likelihood and apply the appropriate Escalation Protocol based on the outcomes :
GSafe Scheduling Information
Scheduling Information
-
Schedule Name: use the following template: 2022 GENERAL H&S AUDIT _SUITE/FACILITY
-
Schedule Name:
-
Audit Name: In GSafe, select "2022 GENERAL H&S AUDIT MANUAL UPLOAD (iAUDITOR)
-
Audit Date
-
The Planned Start Date is the date that the Audit Schedule is uploaded to GSafe
-
Planned Start Date:
-
The Planned Completion Date is the date that Audit Actions must have been entered and assigned in GSafe
-
Planned Completion Date:
-
Workflow for Audit: In GSafe, select 2022 GENERAL H&S AUDIT MANUAL UPLOAD (iAUDITOR)"
-
The Auditor is the person who completes the upload to GSafe
-
Auditor:
-
Position of the Auditor
-
Employee Number of the Auditor
-
In GSafe, select the Person who Owns the risk for the Location/s being Audited
-
Auditee:
-
In GSafe, select the Suite/Location being Audited.
-
Location:
-
In GSafe, the Business Unit will be automatically selected based on the Person being Audited
-
Business Unit:
-
In GSafe, select the person who is responsible for assigning Corrective Actions
-
Corrective Actions Coordinator:
-
In GSafe, select the person who will approve the Audit completion
-
Audit Approver:
-
In GSafe, select the person who will approve the Audit
-
Audit Endorser:
-
Please select "Scott Burnell"
1. COVID Compliance
-
Have you released internal communications on staff noticeboards and through email/teams notifications for all relevant Covid-19 updates?
-
Stay informed
Start by identifying authoritative sources of public health guidance on the epidemic, and stay up to date on officially recommended and mandated actions in the applicable jurisdictions. -
Do you have reliable systems for real-time public health communication with workers?
-
Are your workers aware of the symptoms of Covid-19?
-
Symptoms of coronavirus:
The main symptoms to look out for are:
1. A cough
2. A high temperature
3. A shortness of breath
Cleanliness & Hygiene
-
This may be undertaken by Campus Life in your specific area. How ever it is under the responsibility of the specific area manger to ensure it is done.
-
Have you reviewed and implemented COVID safe cleaning measures to ensure that high risk contact areas and touch points are being regularly disinfected?
-
Have you alerted all staff members to the hand washing protocols with your workplace?
-
Do you have easily available access to Hand Sanitiser, containing minimum 60% alcohol), for all all staff members? Especially high frequency touch points e.g. Entrances, Kitchens, Lifts, printers etc.
Social Distancing
-
Signage is displayed at designated entrance points to inform people that they should: avoid entering the facility if they have a cough or fever; maintain a minimum 1.5 meter distance from one another; and not shake hands or engage in any unnecessary physical contact.
-
Place tape or other markings at least 1.5 meter apart in waiting in line areas indoors and on sidewalks at public entrances with signs directing students and staff to use the markings to maintain distance. Similarly waiting in outside kitchen areas, toilets and printers.
2. WHS Planning
-
The workplace has developed and implemented an WHS Activities Calendar or equivalent. Is there documented evidence of the WHS system?
-
The WHS Activities Calendar or equivalent is reviewed regularly or after relevant milestones or events have occurred. Records demonstrate that the activities identified in the WHS Activities Calendar have been implemented as planned.
-
The WHS Activity Calendar is reviewed and completely updated every twelve months.
3. Communication & Consulting
Notice Boards and Signage
-
At least one WHS Notice Board is established in the workplace.
-
Are WHS Committee Meeting Minutes and Work Group minutes displayed on a notice board? Or Can workers demonstrate how they access these ?<br>
-
Safety signage has been appropriately selected, displayed and maintained
Flow of Information
-
Information communicated from top level management to Schools and institutes.
-
Schools and institutes share information from top level management to workers.
-
Evidence is provided regarding WHS consultation matters with the workers e.g. procedures, safe operating procedures, SWMS, JSA etc
Control Measures
-
When implementing control measures, all persons that may be affected need to be informed of these measures and any changes -- How is this done? (WHS Committee, tool box talks, team meetings or written communications)
-
Is there awareness of procedures for dealing with workplace conflicts?
HSE Committees and Work Groups
-
Do workers participate in HSE Committees and Work Groups?
-
A WHS Committee meeting schedule has been established (minimum quarterly) and is included in the WHS Activities Calendar.
-
Communication forums where WHS is discussed as a standing agenda item have been established and are held on a monthly basis (e.g. staff meetings).
-
Are the outcomes of these meetings disseminated to all workers? Meeting minutes available for evidence of worker consultation.
-
Nominations for a Health and Safety Representative (HSR) or Health and Safety Contacts have been called in the last twelve months and have been offered required training.
WHS Programs
-
Employee Assistance Program awareness raised in the schools and institutes communications?
-
Are there any programs on site that assist with workers personal health and fitness?
4. General Risk & Hazard Management
WHS Documentation
-
An WHS Risk Register has been developed for the school, institute, workshop or workplace,
-
The WHS Risk Register is reviewed and updated on an annual basis or when required
-
Regular WHS inspections are undertaken for all relevant school or workplace areas using the WHS Inspection Checklist
-
The results of the workplace inspections are reviewed by the Workplace Manager or Management WHS Nominee to verify that identified risks are being adequately managed.
-
All relevant WHS hazards for the workplace have been identified and listed in the WHS Risk Register.
-
Control measures for the WHS hazards identified in the WHS Risk Register have been defined following the Hierarchy of Controls. The residual risk level for the WHS hazards identified in the WHS Risk Register for the workplace have been assessed and the risk levels are appropriate.
Permit to Work
-
Is a Permit to Work required (e.g. Heights, Confined spaces?) Was it completed in the last 12 months, filled and signed correctly?
-
General
-
Confined space entry
-
Hot work
-
Working at Height
-
Go to next Section...
Work Environment
-
Is the facility secured to prevent unauthorised access?
-
Work areas are free from excessive rubbish or obstructions. Items are safely stored so they are not at risk of falling or causing injury?
-
Are the Environmental conditions suitable for the tasks being undertaken (i.e. space provided for the task, lighting, temperature, ventilation, flooring, drainage, excessive noise, odour)?
-
Is the facility and furnishings maintained and in good condition (i.e. water leaks, floor damage, pests, structural cracks)?
-
Are workstations ergonomically suitable (e.g. monitors adjusted, common items in easy reach, repetitive postures managed, fully adjustable chairs configured properly)?
-
Are there any housekeeping concerns? (e.g. bins, mould, dust, clutter)
-
Items are safely stored so they are not at risk of falling or causing injury?
Amenities
-
Are toilet facilities available, clean and stocked appropriately?
-
Are hand hygeine services available?
-
Is there a potable water source available?
-
Are tables and food preparation areas clean and well kept?
-
Are there employee lunch facilities available nearby?
Plant and Equipment
Fixed Plant and Equipment
-
Is the equipment being used in compliance with the WHS Regulation. E.g.: guarding, item registration, etc.
-
Site specific equipment has up to date maintenance records and in compliance with WHS Legislation.
-
Daily inspection procedures or pre-start checklists are completed and available. Adequate SWMS / safe operating practices – including monitoring and reviewing controls.
-
High risk equipment (e.g., pneumatic tools, explosive powered tools, lasers) used correctly and maintained.
-
Operators trained and appropriately qualified / licensed / competent. ( license sighted if applicable)
-
Plant and equipment is fit for purpose, in good condition and maintained.
-
Warnings and instructions on plant/equipment are displayed.
-
Emergency stops (where required) are accessible and clearly identifiable.
-
Tag out/lockout procedures are in place for plant with potentially hazardous energy sources (as required).
-
Are tags readily available to workers?
-
Isolation switches are installed on the wall or on the item of plant.
Mobile Plant and Equipment
-
Is there Mobile Plant in the Facility?
-
Mobile plant has safe means of access and egress for operators (e.g., ladders, handrails, non-slip surfaces).
-
Is a process in place to ensure mobile plant is properly maintained, checked, and serviced? Are the records available for this?
-
Is the operating surface appropriate for mobile plant (e.g., sloping ground)?
-
Plant keys and unattended plant kept and parked securely.
-
Safe Work Load for lifting or carrying equipment is displayed and legible and in date (where applicable).
-
Go to next Section...
Electrical Equipment
-
Are electrical installations (plugs, sockets, switches) in good order and compliant with Australian Standards?
-
Electrical equipment and extension leads are current with testing and tagging ?
-
Are double adaptors & piggyback plugs prohibited?
-
Are multi-outlet boards, extension leads and cables mounted clear of benches & floors, at least 300mm?
PPE
-
Workers supplied with suitable PPE and it is used correctly
-
Are workers trained in the use of PPE?
-
Is adequate signage in place where PPE is required?
Manual Handling
-
Are mechanical aids (e.g. trolleys) readily available and used when required?
-
Are frequently used items within easy access between knee and shoulder height?
-
Are heavy items stored at waist height?
-
If applicable, are ladders fit for purpose and industrially rated?
-
Are frequent breaks taken from repetitive tasks?
-
Are manual tasks are being undertaken in accordance with safe manual handling practices
-
Hazardous manual handling tasks have been identified & risk assessed
-
Suitable controls are in place as identified
Confined Spaces and Working in Isolation
-
Confined space(s) are identified and secured against unauthorised entry.
-
Confined space permits, risk assessments are completed and signed off
-
Are there procedures and support for working in isolation, working alone and working from home?
-
Adequate means Means of communication are provided when undertaking remote and/or isolated work activities
Working From Heights
-
Have tasks at the site that could result in a fall from one level to another been identified?
-
Have hazards associated with work-at-height tasks been identified, risk assessments completed and risk controls identified?
Slips Trips and Falls
-
Is the tread on the floor appropriate for the activity being conducted?
-
Are walkways clear of extension cords and litter?
-
Are there any floor variations, if so demarcated?
Work Related Driving
-
Is there work related driving activities undertaken by the workplace?
-
Are you familiar with appropriate driving policy and procedure
-
The individuals driving have appropriate training and license?
-
Does the workplace have an appropriate travel plan and / or risk assessment?
-
For Griffith Vehicles are the maintenance and service records available for review? And if they are up to date ?
-
-
Go to next Section...
Traffic Management
-
Is a Traffic Management Plan Applicable?
-
The site has an appropriate traffic management plan
-
The site traffic management risk assessment has been reviewed and updated annually if needed
-
Appropriate controls are in place to reduce traffic risk (photo for each control)<br>• Speed limit/Signage<br>• Separation barriers<br>• Pedestrian walkway/crossing/signage<br>• Traffic flow direction<br>• Convex mirror<br>• Loading bay/dock and signage<br>• Parking Facilities
5. Specialist Risk & Hazard Management
Biosafety Hazards
-
Are there identified or potential Biosafety Hazards normally present within the facility?
2.0 Research Material and Project Details
2.1 Research Material
-
3.1.1 What type(s) of samples are used within this facility?
- Risk Group 1 Bacteria
- Risk Group 2 Bacteria
- Risk Group 2 Parasites
- Viruses
- Human/Animal Tissue Culture
- Plant Work incl Pathogens
- Invertebrate (Drosophila)
- Human/Animal Diagnostic Specimens
- Small Animals (Rats/Mice)
- Prions
- Aquatic Organisms
- Jelly Fish
- Sharks
- Soil
2.2 Project Details
-
2.2.1 If applicable, what type of OGTR/UBC projects are approved to be undertaken within the facility? Please list the projects currently approved in GSafe.
- Exempt dealings
- Notifable Low Risk Dealings
- DNIR
- DIR
- N/A
-
2.2.1 (i) Are all UBC or regulator issued approvals current?
-
2.2.1 (ii) Are training records available to show users have read and understand the conditions outlined in the UBC memos? For DNIR license this also includes people autoclaving waste.
2.3 Other Biological Dealings (other than GM)
-
Other Biological Dealings include any of the following:
1. Risk Group 2 Organism (10L or greater)
2. Risk Group 2 with special precautions
3. Risk Groups 3 or 4
4. Infectious/potentially infectious animals, tissues or fluids (involving microorganisms of the categories mentioned above)
Unscreened specimens?
i.e. human tissue or body fluids that are known to contain microorganisms listed above, or have not been screened for infectious disease; animal tissues or body fluids that could contain zoonoses or have not been screened for such.
5. Poisonous or venomous animals?
6. Biological toxins (excluding toxoids)?
7. Security Sensitive biological Agents (SSBAs)
8. Biological material on the Defence Strategic Good Lists (DSGL) -
2.3.1 Are Other Biological Dealings undertaken in the facility?
-
2.3.1 (i) Have the dealings being registered in GSafe? If so please enter reference numbers
3.0 Personnel and Training Records
3.1 Personnel
-
3.1.1 Is a list of researchers including students who access the facility kept and readily available?
-
3.1.1 (i) When was the list last reviewed?
-
3.1.1 (i) Reason for no list:
3.2 Induction and Training Records
-
3.2.1 What on-line training modules are required in the facility?
- Annual Fire Safety
- Biosecurity
- Gas Cylinder Safety
- General Biosafety
- Genetic Biosafety
- Health & Safety Induction
- Laboratory & Workshop Safety
- General Chemical Safety
- Animal Ethics
-
3.2.2 Is a procedure in place to ensure the on-line modules are completed? Please outline procedure.
-
3.2.3 Is a facility induction provided to everyone working in the facility?
-
3.2.3 (i) What does the facility induction cover?
- Facility work practices
- Decontamination including decontamination and safe waste handling
- Emergency procedures including spill
- Project Specific Training
- UBC Memo training
- Safe handling infectious/potentially infectious biological material
-
3.2.3 (i) Are training records available for the Facility Induction?
-
3.2.3 (i) (i) Are these records kept up-to-date?
-
3.2.4 Do facility users receive project specific training? Please outline process for ensuring all personnel are adequately trained.
-
3.2.5 Do contractors/visitors to the facility have a safety induction before commencing work in the facility or are they accompanied?
3.3 Risk Assessments
-
Risk Assessments are required for projects, activities and equipment.
Note: It is imperative that risk assessments are developed and documented. Where there are no risk assessments (evidence of risk assessments) in place, please ensure they are developed and approved as soon as practicable. -
3.3.1 Are risk assessments on activities details available? For example, preparing gels and DNA extractions.
-
3.3.2 Are risk assessments on project details/activities available? For example, exempt dealings and NLRD.
-
3.3.3 Are risk assessments on equipment available? For example, use of the autoclave and Gel Doc.
-
3.3.4 The relevant workers have read and understood the risk assessments?
4.0 Storage and Transport
4.1 Storage of Biological Material
-
4.1.1 Is biological material(s) stored "inside" the facility?
-
4.1.1 (i) Where is the material stored?
- Cupboard
- Fridge
- -20C Freezer
- -80 Freezer
- Walki-in Cold Room
- Dewar
- IVCs or other animal housing devices
-
4.1.1 (ii) Is the storage unit labelled to clearly show the name and contact details of the person responsible for the material?
-
4.1.1 (iii) Is the storage device labelled with a biohazard label?
-
4.1.1 (iii) Is an inventory available for all biological material present in the facility?
-
4.1.1 (iii) (i) What information is displayed in the inventory? Please photograph the inventory.
- Date
- Description
- Licence Number
- Amount/Number
- Location
- Owner
- GM Characteristic
- Other
-
4.1.1 (iii) (i) Is the biological material labelled in a way that allows it to be easily identified? For GMOs the material should be labelled so that it can be easily identified as a GMO and distinguished from non-GMO material.
-
4.1.1 (iii) (v) What information does the label contain?
- Description of material
- Owner
- Unique reference number
-
A detailed Inventory of all GMOs must be kept and made available to the UBC.
-
4.1.2 Is biological material(s) stored "outside" the facility?
-
4.1.2 (i) Where is the material stored:
4.2 Transport of Biological Material
-
4.2.1 Is biological material transported external to the facility?
-
4.2.1(i) Are users trained in the transport of biological material?
-
4.2.1 (i) (i) Are training records available?
-
4.2.1 (i) (ii) Are the training records up to date?
-
4.2.1 (ii) Is there a Transport SOP?
-
4.2.1 (ii) (i) What aspects does the SOP cover?
- Packaging
- Spill Kit
- Route
- Floor Plan
- Contacts
-
A Transport SOP must be developed and users trained in its operation.
Please consult Biosafety Advisors if you require assistance in this matter. -
4.2.3 (i) What are the physical attributes of the container used to transport biological material?
- Solid Outer Wall
- Solid Inner Wall
- Flexible Outer Wall
- Flexible Inner Wall
- Lockable
- Clip Lock
- Handle
-
4.2.3 (ii) What labeling is attached to the container?
- Contact (Sender)
- Address (Sender)
- Contact (Recv)
- Address (Recv)
- BioHazard Label
- GMO Label
- Other warnings
-
4.2.3 (iii) Are the transport containers decontaminated after transport is completed?
-
4.2.3 (iv) Is a record maintained of the type and quantity of biological material transported?
5.0 Facility Decontamination, Spill Procedure and Waste Disposal
5.1 Decontamination
-
5.1.1 Is there a documented SOP in place for all decontamination practices?
-
5.1.2 Is all equipment, pens, protective clothing, cages or bedding contaminated with or suspected to be contaminated with regulated material(s) decontaminated by an approved method(s) before being removed from the facility? <br><br>N.B: In this context, appropriate/approved methods include: pressure steam sterilisation (autoclaving); chemical treatment; or any other method approved in writing by the relevant governing body.
-
5.1.2 (i) How is the material decontaminated?
- 0.5% to 10% Sodium Hypochlorite
- Neat Sodium Hypochlorite
- 70%w/w Ethanol
- 80%v/v Ethanol
- Virkon
- Viraclean
- Other
- None
-
5.1.3 Is there a supply of disinfectants available in the facility which are effective against the biology material handled in the facility?
-
5.1.3 (i) Are disinfectants clearly labelled with contents and expiry date if applicable?
-
5.1.4 Are all work benches, surfaces & equipment where procedures have taken place decontaminated immediately after any spills and when work is completed?
5.2 Biological Spill SOP and Spill Kits
-
5.2.1 Is there a biological spill SOP in Place?
-
A biological spill SOP must be developed for all facilities that undertake work with Biological agents regardless of whether they are classified as regulated or high-risk material.
-
5.2.1 (i) Does the spill SOP cover minor and major spills?
-
5.2.1 (ii) When was the biological spill SOP last reviewed?
-
5.2.1 (iii) Which disinfectant is used?
- 0.5% to 10% Sodium Hypochlorite
- Neat Sodium Hypochlorite
- 70%w/w Ethanol
- 80%v/v Ethanol
- Virkon
- Viraclean
- Other
- None
-
5.2.1 (iv) Describe disinfectant including concentration and contact time:
-
5.2.1 (v) Are the decontamination agents referenced in the SOP appropriate for the biological material?
-
5.2.2 Is there a biological spill kit in the facility?
-
5.2.2 (i) Is the location easily identified?
-
5.2.2 (ii) What are the contents of the spill kit?
- Decontamination agent
- Gloves
- Mask
- Absorbent material
- Autoclave bags
- Brush
- Other material
-
5.2.2 (ii) (i) Describe other material:
-
5.2.2 (iii) Which disinfectant is used in the spill kit?
- 0.5% to 10% Sodium Hypochlorite
- Neat Sodium Hypochlorite
- 70%w/w Ethanol
- 80%v/v Ethanol
- Virkon
- Viraclean
- Other
- None
-
5.2.2 (iii) (i) Is the disinfectant within its expiry date or contain a date of preparation?
-
5.2.2 (iv) Does the agents present in the spill kit match the SOP?
5.3 Disposal of Facility and GMO Waste
-
5.3.1 Are bins used for general waste located within the facility?
-
5.3.1 (i) Are these bins suitably marked to indicate that no waste other than general waste is permitted?
-
5.3.2 Does the facility produce clinical and related waste (CRW)?
-
5.3.2 (i) How is clinical waste segregated?
- Clinical Waste Bags
- Clinical Waste Bins
- Cytotoxic bins
- CRW Bags in Frames
- CRW Bags in Containers
-
5.3.2 (ii) Is waste being correctly segregated in the facility?
-
5.3.2 (iii) Are users familiar with safe handling processes when dealing with clinical and related waste?
-
5.3.2 (iv) Is the exterior of the waste bin clearly marked clinical waste?
-
5.3.2 (v) At the time of the inspection, were the clinical waste bins no more than 2/3 full?
-
5.3.2 (vi) Is liquid and solid waste decontaminated prior to being disposed of in the CRW stream?
-
5.3.2 (vi) (i) Is it safe to dispose of the waste via the CRW stream without first being decontaminated?
-
5.3.2 (vi) (i) (i) Please describe issues with directly disposing into the CRW stream:
-
5.3.2 (vi) (i) What method(s) are used to decontaminate the waste?
- Autoclave (15 mins)
- Autoclave (20 mins)
- Autoclave (30 mins)
- Autoclave (>30 mins)
- Sodium Hypochlorite (1 %)
- Sodium Hypochlorite (5 %)
- Sodium Hypochlorite (Straight)
- Other Chemical
- Dry Heat (160C)
-
5.3.2 (vi) (i) (i) Describe the chemical including concentration and contact time:
-
5.3.3 Is waste generated within the facility from activities involving regulated material?
-
5.3.3 (i) Is there a SOP available for the disposal of regulated waste?
-
An SOP outlining all steps on waste disposal of regulated material must be written and kept in the facility.
-
5.3.3 (i) (i) Is the Waste SOP up-to-date and covers all aspects of the waste treatment?
-
5.3.3 (ii) Is regulated material(s) waste disposed of immediately (i.e. disposed of same day it is generated)?
-
5.3.3 (ii) (i) Where waste cannot be disposed of immediately, is it stored in lidded bins/containers of an appropriate size which are lack and pest proof?
-
5.3.3 (iv) Is regulated material(s) waste rendered non-viable prior to disposal?
-
5.3.3 (iv) (i) How is regulated waste rendered non-viable?
- Autoclave (15 mins)
- Autoclave (20 mins)
- Autoclave (30 mins)
- Autoclave (>30 mins)
- Sodium Hypochlorite (1 %)
- Sodium Hypochlorite (5 %)
- Sodium Hypochlorite (Straight)
- Other Chemical
- Dry Heat (160C)
-
5.3.3 (iv) (i) Where viable regulated material(s) waste is being transported out of the facility, is there a documented SOP in place?
-
5.3.3 (iv) (ii) Where viable regulated material(s) waste is being transported out of the facility, are records/logs maintained and readily available?
-
5.3.3 (v) (iii) Where viable regulated material(s) waste is being transported out of the facility, is it bagged and placed in an unbreakable container with a secured lid?
-
5.3.4 If water waste is sewerable, are documents indicating this in the SOP?
-
5.3.5 Are sharp bins being used correctly and no more than 2/3rd full?
6.0 Facility Equipment
6.1 Pressure Steam Sterilizer
-
6.1.1 Does the facility contain a pressure steam steriliser?
-
6.1.1 (i) Is an autoclave used for the decontamination of waste?<br>
-
6.1.1 (i) (i) How is waste disposed?
- Clinical Waste Stream
- Chemical
- General Waste
-
6.1.1 (i) (i) If an autoclave is utilised and located elsewhere please provide the following:
-
6.1.1 (i) (ii) Location of autoclave:
-
6.1.1 (i) (iii) Latest UBC Inspection Report Number: 18/
-
For Each Autoclave Please complete the following.
Autoclave
-
6.1.1 (i) What is the decontamination cycle?
-
6.1.1 (ii) Is the temperature of each cycle monitored?<br><br>i.e. through use of a thermocouple and recorder; a maximum thermometer; chemical indicator or readings from the autoclave panel?
-
6.1.1 (iii) How are processed loads differentiated from unprocessed loads?
-
6.1.1 (iv) Is a log of autoclave use kept and available?
-
6.1.1 (v) (i) What information does the log contain?
- UBC Record Form
- Date
- Operator
- Temperature/Time
- Items
- Successful Run
- Licence Information
-
6.1.1 (vi) Is monthly checking of efficiency with biological or chemical indicators undertaken?
-
6.1.1 (vi) (i) What records are present?
- If succesful
- Date test carried out
- name of person carrying out the test
- None
-
6.1.1 (vii) Has the autoclave undergone annual testing and calibrating?
-
6.1.1 (ix) (i) Is the autoclave used for the decontamination of Regulated or High Risk Biological Waste?
-
Autoclave is not to be used for decontaminating Regulated or High Risk Biological Waste until is has been tested and calibrated to ensure that successful decontamination of biological material can be guaranteed.
If you need to decontaminate such material, please ensure that you use another autoclave that has undergone annual testing and calibration. -
6.1.2 Are users trained in how to correctly load the autoclave and the hazards associated with heat and steam?
-
6.1.3 Is protective clothing, including heat-insulating gloves, readily available?<br><br>i.e. for use when loading and unloading sterilisers.
6.2 Aerosol Containment Equipment
-
Wherever there is work with regulated material or high risk biological agents that can produce aerosols, equipment that can contain said aerosols must be installed and utilised.
-
6.2.1 Is there work being undertaken that can produce aerosols
-
6.2.1 (i) What types of aerosol containment devices are present?
- BSC Class II
- Laminar Flow
- PCR Clean Hood
- Animal Change Station
-
6.2.1 (i) (i) Is the BSC suitably positioned (in the opinion of the inspector) so that any room ventilation will not interfere with neither the air intake or exhaust of the BSC?
-
6.2.1 (i) (i) (i) Please describe the risks observed at the time of the inspection.
-
6.2.1 (i) (ii) Has the BSC been serviced in the past 12 months? Please take a photograph of the certification label/s
-
6.2.1 (i) (ii) (i) Please provide reason[s] for the BSC being out of specified service period.
-
6.2.1 (i) (i) Has the Laminar Flow/PCR Clean Box/Animal change station been serviced in the past 12 months?
-
6.2.1 (i) (i) (i) Is there a current certification label present?<br><br>Please take photograph of said label.
-
6.2.1 (i) (i) (i) Reason for no current servicing of Laminar Flow/PCR Clean Box:<br><br>Please Note: If BSC is used for containment of GMO or High Risk Biological agents, it must be serviced annually.
-
6.2.2 Is there an Individually Vented Cage System in the facility?
-
6.2.2 (i) Has the Individually Vented Cage System been serviced in the past 12 months? Please take a photograph of the certification label.
-
6.2.2 (i) (i) Reason for no current servicing of Individually Vented Cage System?<br><br>Please Note: If BSC is used for containment of GMO or High Risk Biological agents, it must be serviced annually.
-
6.2.2 (i) (ii) Is there a user of the Individually Vented Cage System present in the facility and able to comment on their use of the equipment?
-
6.2.2 (i) (ii) (i) What is the process for decontaminating the surfaces of the Individually Vented Cage System .
- Ethanol on surfaces
- 70% w/w Ethanol
- 80% v/v Ethanol
- Sodium Hypochlorite
- 1% Sodium Hypodchlorite
- Clean beneath shelf
6.3 Centrifuge Equipment
-
6.3.1 Does the facility contain centrifuges?<br><br>N.B: generates force in excess of 2G, and as such presents a greater risk to health and safety.
-
6.3.1 (i) If centrifuges are used and not located in the facility, please outline details of the location.
-
6.3.1 (i) (i) Location:
-
6.3.1 (i) (ii) UBC Inspection Report Number:
-
For each Centrifuge, please add a new field.
Centrifuge
-
Please take an image showing the brand, model and Serial number of each centrifuge
-
6.3.1 (i) What is the condition of the centrifuge?
- Clean/Compliant
- Dirty interior
- Damaged Rotor
- Components missing
- Inoperable
-
6.3.1 (iii) Are log books for usage located near instrument and do they indicate maintenance dates/results?
-
6.3.1 (iii) (i) What records are kept?
- Complete (Date, Speed, Time, Operator)
- Limited (Date, Time, Operator)
- Incomplete (Date, Time)
-
Record of centrifuge use is important as a means of ensuring accountability of use and for indicating when maintenance is due.
Please ensure that a log book system is put in place ASAP -
6.3.1 (iv) Which of the following checks are performed by the users of the centrifuges in the facility?
- Rotors
- Cleanliness
- Breakages of tubes
- Leaks
- Balancing
- No Checks
-
It is essential for users to check the condition of the centrifuge both before loading and prior to removing tubes from the centrifuge.
-
6.3.1 (v) Centrifuge Maintenance program:
- Rotors/Buckets Decontamination.
- Maintained per Suppliers Instructions
- Regular Cleaning
- No Preventative Maintenance
-
It is suggested that a preventative maintenance program be implemented.
7.0 Facility Work Practices
7.1 Personal Protective Clothing & Equipment
-
7.1.1 What is the standard PPE required in the facility?
- Lab Coat (Front Closing)
- Gown (Rear Fastening)
- Enclosed Foot wear.
- Eye Protection
- Hair Net
- Gloves
- Overshoes
- Scrubs
- Surgical Mask
- P2 Mask
- Other
-
7.1.1 (i) Describe Other PPE:
-
7.1.2 Is PPE removed when leaving the Facility?
-
7.1.2 (i) What reason[s] are provided for wearing PPE when leaving the Facility?
- Moving within Building to another Facility
- Permitted according to SOP
- No reason
-
7.1.2 (i) (i) Describe reasoning for wearing PPE outside facility:
-
7.1.3 Is specialist PPE required when working in the Facility?
-
7.1.3 (i) Please list specialist PPE that is required in the facility.
-
7.1.4 Is PPE hung in a manner that prevents contamination of surfaces in direct contact with the users street clothes?
-
7.1.4 (i) How is PPE hung/stored?
- Individual Hooks
- Coat Hangers
- Coats overlaping
- Hung seperate
- On chairs
- Disposable (Drawers)
-
7.1.5 How often is PPE laundered?
- Weekly
- Monthly
- Bi-Monthly
- When soiled
- Never
- Daily
- Fortnightly
- Quarterly
- Every 6 months
-
7.1.5 (i) Is disposable PPE used in the facility?
-
7.1.5 (i) (i) When is disposable PPE disposed of?
- After each use
- Weekly
- Fortnightly
- Monthly
- Not Disposed
- When soiled
-
Disposal PPE must be disposed of once it becomes contaminated or when its integrity is compromised.
Please modify your PPE SOP to reflect this. -
7.16 Are potentially contaminated laboratory gowns decontaminated before laundering?
7.2 Non-Laboratory work.
-
This relates to any work not directly related to bench work.
-
7.2.1 Is there a dedicated reading/writing area provided within the facility?
-
7.2.1 (i) Is the space only used for reading/writing/data entry and no work with biological agents is undertaken?
-
7.2.1 (ii) At the time of the inspection, was the area in a clean and tidy state?
-
7.2.1 (ii) (i) please select from pick-list the issues that were observed?
- Untidy.
- Dusty/Dirty
- Lab material present
- Excessive reading material
- Food/Drink items
- Personal posessions
- Other (Please explain)
-
7.2.2 Are there any obvious manual handling issues observed or brought up by the users?
-
7.2.2 (i) List issues raised by users:
7.3 Other Work Practices
-
7.3.1 Are there any specialist Work Practices associated with this facility?
-
Specialist Work Practices
Work Practices
-
7.3.1 (i) What is the Work Practice:
-
7.3.1 (ii) Provide information on Work Practices.
8.0 Animal Facility
-
8.1.1 Is this an Animal facility?
8.1 Animal Facility Requirements
-
8.1.1 (i) Does the facility have a dedicated anteroom or room that acts as an anteroom?
- Dedicated Anteroom
- Anteroom to each Animal Room
- Lab used as Anteroom (Exemption)
- Corridor used as Anteroom (Exemption)
- Other (No Exemption)
-
8.1.1 (ii) Is there a documented system of accounting for the number of animals in the facility?
-
8.1.1 (ii) (i) Which system(s) is used to account for the number of animals in the facility?
- Genotrack
- Logbooks
- Electronic Records
- None
-
8.1.1 (iii) Is animal work (other than housing) undertaken in the facility?
- Work on Live Animals
- Work on Deceased Animals
- Work on Animal Tissues
-
8.1.1 (iii) (i) Are animal ethics approvals in place?
-
8.1.1 (iii) (ii) Are animal carcasses and tissues disposed of in an appropriate and approved manner?
-
8.1.1 (iii) (i) Are animal ethics approvals in place?
-
8.1.1 (iii) (ii) Are animal carcasses and tissues disposed of in an appropriate and approved manner?
8.2 Animal Facility Work Practices
-
8.2.1 Is work with a viable GM Animal undertaken in the facility?
-
No Further questions on this section.
-
8.2.1 (i) Are the Animals housed in Individuality Ventilated Cages?
-
8.2.1 (ii) How are the GM Animals Labeled?
- Tattoos
- Notchings
- Cage Labels
- Bandings
- None
-
8.2.1 (iii) Is animal handling performed in a way to minimise the chance of escape?
-
8.2.1 (vi) Are GM Micro-organisms used with animals?
-
8.2.1 (vi) (i) Are animals autoclaved prior to disposal or removal from the facility for disposal?
-
8.2.1 (vii) Is there a documented SOP for dealing with the escape of an animal WITHIN the facility?
-
A detailed SOP for dealing with unintentional release of a GMO must be produced and be available in the facility.
-
8.2.1 (vii) (i) What sections are covered in the SOP?
- Method to be used.
- Reporting (GSafe)
- Reporting (UBC)
- Decontamination Method
- Records
-
8.2.1 (viii) Is there a documented SOP for dealing with the escape of an animal OUTSIDE the facility?
-
A detailed SOP for dealing with unintentional release of a GMO must be produced and be available in the facility.
-
8.2.1 (viii) (i) What sections are covered in the SOP?
- Method to be used.
- Reporting (GSafe)
- Reporting (UBC)
- Decontamination Method
- Records
9.0 Emergency Procedures & Equipment
-
This section covers Emergency Procedures in the Facility.
9.4 Safety Showers and Eye Wash
-
9.4.1 Does the Facility have a Safety Shower/Eye Wash Station?
-
9.4.1.1 Please indicate if plumbed or single-use packs:
-
9.4.1.1. (i) Are the single use packs clearly labelled and visible to users within the facility?
-
9.4.1.1 (ii) Are the Single use packs within their expiry date?
-
9.4.1.1 (i) Is the Eye Wash and safety shower Station, tested as per the legislative requirements?
-
9.4.1.1 (ii) Enter date. mm/yy of last test.
-
9.4.1.2 Is the Safety Shower and Eye Wash Station free from obstructions?
-
9.4.1.2 (i) List the obstructions?
- Boxes/Containers
- Waste Bins
- Cleaning Equipment
- Other items
-
9.4.1.2 (i) (i) Describe obstructions,
-
Photograph obstructions
9.5 Emergency Procedures - Biosafety
-
9.5.1 Does the facility hold regulated material?<br><br>GMO from a licence dealing
-
9.5.1.1 Has a documented risk assessment been completed for the escape of any regulated biological material in an emergency event (e.g. natural disaster, deliberate action, accident or fire)?
-
A documented Risk Assessment must be complied on the likelihood of escape or loss of regulated material from a facility.
-
9.5.1.1 (i) When was this Risk Assessment last reviewed?
-
9.5.2 Has a Contingency Plan been compiled to address issues such as unavailability of facility; loss of power; loss of refrigeration; loss of containment?
-
A documented Contingency Plan must be complied to address issues such as unavailability of facility; loss of power; loss of refrigeration; loss of containment.
Pleas ensure that one is written as soon as possible. -
9.5.2.1 When was this Contingency Plan last reviewed?
10.0 Facility Fitting, Infrastructure and Cleanliness
10.1 General Conditions
-
10.1.1 How are the windows secured?
- Fixed panes
- Permanently closed
- Key Locked
- No windows
-
10.1.2 Is a wash basin fitted with hands-free operation or an alternative method of decontaminating hands available?
- Hands Free Levers
- Electronic Activation
- Foot Control
- Normal Tap
- No Basin
- Hands free decontamination unit
-
10.1.2 (i) Are hand basins clean and tidy?
-
10.1.2 (ii) Is paper toweling provided for drying hands after washing?
-
10.1.2 (iii) Which decontamination agent is used?
- Chlorohexidene
- Deb Disinfectant
- Commercial Soap
- Alcohol based foam
- Other
-
10.1.2 (iii) (i) Is the decontamination agent in date?
-
10.1.2 (i) Is there access to a hands-free decontamination unit?
-
10.1.2 (i) (i) Which decontamination agent is used?
- Chlorohexidene
- Deb Disinfectant
- Commercial Soap
- Alcohol based foam
- Other
-
10.1.2 (i) (i) (i) Is the decontamination agent within its expiry date?
-
10.1.2 (i) Please describe agent.
-
10.3.4 Are drains suitably screened?
- Coarse screen (Floor)
- Fine Screen (Floor)
- Full Covers
- No covers
- No Floor Drains
-
10.3.4 (i) Please take photographic evidence:
10.4 Additional requirements for Invertebrate Facilities.
-
10.4.2 Is this a PC2 Invertebrate Facility?
-
10.4.2 (i) Is entry/exit to the facility via a dedicated anteroom?
-
10.4.2 (ii) Are facility access doors self closing?
-
10.4.2 (iii) Are the facility doors designed to prevent the escape of animals contained therein?<br><br>e.g. by the use of seals on the edges of all doors.
-
10.4.2 (iv) Are all joints between structural components sealed and impenetrable to animals contained in the facility?
-
10.4.2 (v) Are measures in place for monitoring and controlling the escape of invertebrates out of the facility?<br><br>i.e. measures for monitoring and controlling invertebrates may include using traps, visual inspections, use of mirrors/visual aids, accounting procedures for live and trapped invertebrates and methods for attracting invertebrates away from the door.
-
10.4.2 (v) (i) Is there a documented SOP for these monitoring measures available on request?
-
10.4.2 (v) (ii) Are records/logs of measures undertaken available on request?
10.5 Ventilation
-
10.5.1 What is the condition of the ventilation registers?
-
10.5.1 (i) Please described the concerns with the ventilation registers, including location (e.g. room number):
10.6 Environmental and Ventilation Requirements
-
10.6.1 Was inward flow of air into the facility observed or tested?
-
10.6.1 (i) Inward air flow was observed:
- Doors
- Windows
- Ventilation Registers
- Drains
- Not tested.
-
10.6.1 (i) Inward air flow was not observed:
-
10.6.2 Do users indicate any concerns with the facility environment?<br><br>For example, concerns with noise levels, lighting relevant to tasks undertaken, temperature, ventilation.
-
10.6.2 (i) Please list issues or concerns of users:
10.7 Backflow Prevention
-
10.7.1 Is the water supplied to the laboratory provided with back flow prevention?
-
10.7.1 (i) If installed at time of certification is it maintained and tested annually (if applicable)?
-
10.7.1 (i) Where no back-flow prevention device is in place, are there risk assessments documented and available?
10.8 Pest Control
-
10.8.1 Were any pests sighted during inspection?
-
10.8.1 (i) What pests or evidence of, present within the facility? (Provide evidence)
- Live pests
- Dead pests
- Ants
- General Insects
- Cockroaches
- Flies
- Reptiles
- Faeces
-
10.8.2 Are strategies in places to address Pest Control?
-
10.8.2 (i) What Strategies are in place?
- Pest Log
- External Pest Control
- Knock-down Spray
- Active Monitoring
- Other
-
10.8.2 (i) (i) Is the Pest Log entries up-to-date?
-
10.8.2 (i) (ii) Are the external Pest Control Treatment Records available?
-
10.8.2 (i) (i) Please describe Other Pest Strategy:
-
Go to next Section...
Biosecurity Hazards
-
Are there identified or potential Biosecurity Hazards normally present within the facility?
-
Is the Facility an Approved Arrangement (AA) Site?
Section 1.0 Security
-
1.1 Is an appropriate biosecurity sign displayed where goods subject to biosecurity control are stored or handled? NOTE: Cardboard and paper signs are not acceptable. Where new signs are being produced, they should use "Biosecurity" not "Quarantine".
-
1.1.1 Is a biosecurity sign secured on a building(s), racks, fences, gates and/or doors, and visible at all times?
-
1.1.2 Is the biosecurity sign made to state: ‘Biosecurity Area, Microbiological Facility - BC1 Facility - Authorised Persons Only, No Entry or Removal of Goods, Penalties Apply, (Biosecurity Act 2015)’; or as directed for specific biosecurity operations?
-
1.2 Has the name, designation/position title and contact details of the new nominated Griffith University staff member responsible for the security of the AA been supplied to the Department?
-
1.3 Is the name and telephone number of the Facility Manager or other responsible person[s] displayed near all access doors?
-
1.4 Does the facility have physical security measures in place to prevent unauthorised access to goods subject to biosecurity control?<br>NOTE: Physical sceurity measures may include a fence/gate/door that is lockable at all times when personnel are not present
-
1.5 Is there a procedure/process in place to immediately notify the Department of any incidents which could significantly compromise the biosecurity security of the facility? NOTE: This may include structural damage, electrical breakdowns, escape or unauthorised entry and the removal of material subject to biosecurity control.<br>
Section 2.0 Requirements to Maintain Approval
-
2.1 Has there been any changes to the AA Site operating procedures or arrangements?
-
The Department must be notified of any changes within 30 days.
-
2.2 Have there been any structural and/or fitting changes to the AA site?
-
2.2.1 Has a change significantly affected the overall containment system, including structural changes to 40% of the building? NOTE: A change that significantly affects the overall containment system requires re-certification. <br>
-
2.2.2 Has the Department been notified in writing within 15 working days of any alternations to the AA site management arrangements?
-
2.2.3 Where structural changes have been made to the AA site, has the Department been provided with a written statement describing the details of the alternations?
-
2.2.4 If requested, has the Department been provided with documented evidence of compliance with AS/NZS 2982.1 and 2243.3 when additions or modifications have been made to the facility?
Section 3.0 Biosecurity area and Isolation
3.1 Biosecurity Area
-
3.1.1 Is the biosecuirty area of a size commensurate with the proposed quantity of goods being handled?
-
3.1.2 Are biosecurity areas and goods subject to biosecurity control accessible for the purpose of the inspection? NOTE: Accessible means goods must be able to be inspected as directed by a biosecuirty officer. Generally, block stacking will not be regarded as being accessible?
3.2 Isolation
-
3.2.1 Are the biosecurity areas separate from other operations within the AA site?<br><br>NOTE: Examples of how biosecurity area separation can be achieved include: isolation from main thoroughfares with yellow lines, structural separation, a lockable room or building, a person proof security fence, separate benches or similar structure.
-
3.2.2 Are cool/cold rooms, refrigerators, freezers or other storage units located outside the area were biosecurity work is undertaken? <br>NOTE: Where this is necessary, the AA site will need to have more than one biosecurity area. <br>
-
3.2.2. (i) Is the additional biosecurity storage area located outside the designated facility within the one physical site? NOTE: To be within one physical site the facility must be within the same common boundary as the approved storage area and must be approved under the one organisation or company.
-
3.2.2. (ii) Where material subject to biosecurity control is stored outside the designated AA site, is there a transfer procedure in place to ensure the safe movement of goods subject to biosecurity control?
-
3.2.2 (iii) For a biosecurity storage area located outside the building that houses the AA site:<br>- are the areas fully enclosable spaces contained within walls, doors, windows, floors and ceilings; and<br>- are doors and windows lockable and secure;<br>-and are the floors impermeable
-
3.2.2 (iv) Where a biosecurity area is outside or separate to the area where work is undertaken, is the type of biosecurity area (e.g. refrigerator, freezer) stated on the scale drawings?
-
3.2.2 Are goods subject to biosecurity unpacked within the containment boundary?
-
3.2.3 Is the AA managed to ensure that effective separation is maintained between cleared imported goods, domestic goods, imported goods awaiting release from biosecurity control, and (in the case of Departmental approved dual import and export AA site), exported goods?
-
3.2.3 (i) How is effective separation achieved?
- An impervious barrier
- Other Departmental Approved methods
-
3.2.4. What approved method is used to differentiate domestic goods from biosecurity goods? NOTE: The use of a method must be approved by the Department.
- Sealed Containers
- Storage in separate rooms
- Remain consolidated within the shipping container
- Plywood, sheet metal or heavy gauge plastic sheeting that provides complete & unbroken physical separation between consignments
- Double plastic wrap including a space separation between consignments of 1.2 metres
- Other
-
3.2.4 (i) (i) Please specify the "Other" method:
-
3.2.5 Is there a documented procedure in place should cross-contamination occur? NOTE: Should cross-contamination occur, all goods shall be treated as goods subject to biosecurity control. <br>
-
3.2.6 In addition to the Department’s requirements, do Import Permit conditions and inspection procedures for some commodities apply?
Section 4.0 Biosecurity Records and Traceability
4.1 Training Records
-
4.1.1 Have persons undertaking work with biosecurity goods undertaken the appropriate third party training?
-
4.1.2 Have all persons associated with AA sites and goods subject to biosecurity control been deemed as a ‘fit and proper person', and confirmed their status in writing?
-
4.1.3 Is there an agreement in place with the Department for training and/or electronic initiatives?
4.2 Biosecurity Records
-
4.2.1 Is a scale drawing, with dimensions and locations of biosecurity area(s) readily available?
-
4.2.2 Is a system in place to identify and date the arrival of all goods subject to biosecurity control?<br>NOTE: The system must include tracking the creation of direct and indirect derivatives, and tracking and control of the distribution of goods/derivatives.
-
4.2.3 (i) Is the system maintained and up to date?
-
4.2.4 For each consignment of goods subject to biosecurity control is records showing the receipt and holding which includes the date of arrival, type (e.g. species, scientific names), country of origin and total quantities received available?
-
4.2.5 For each consignment of goods subject to biosecurity control is a record kept of the location or part of the facility where the goods are stored?
-
4.2.6 For each consignment of goods subject to biosecurity control is a record kept of the import permit or import permit number?
-
4.2.7 For each consignment of goods subject to biosecurity control is a record of the biosecurity directions (e.g. entry and release) available?
-
4.2.8 Can goods subject to biosecurity control be clearly reconciled against import permits, biosecurity directions and other documents such as shipper's declarations, treatment processing etc?
-
4.2.9 Are goods subject to biosecurity control held in a sealed primary container at all times when in the biosecurity containment storage area/unit?
-
4.2.10 Do administration and documentation requirements provide assurance that there are adequate controls in place?
4.3 Transport of Biosecurity Material
-
4.3.1 Has biosecurity material been transferred to a co-located facility?
-
4.3.1 (i) Is a transfer procedure for the safe movement of goods subject to biosecurity control between co-located facilities in place?
-
4.3.1 (i) (i) What details are included in the documented transfer procedure?
- Transport container
- Route to be taken
- Emergency contact details
-
4.3.1 (i) (i) Please specify the "other relevant information".
-
4.3.1 (ii) Are the biosecurity goods transported in a primary container/receptacle that is shatter proof, crush resistant and prevents the spillage, loss or escape of the goods subject to biosecurity control?
-
4.3.1 (iii) What records are kept for biosecurity material that has been transferred to a co-located facility?
- Name and type of AA site
- Date of movement
- Import permit
- Description of goods transferred
- Quantity of goods transferred
-
4.3.2 Has biosecurity material been transferred to or received from a non co-located AA site?
-
4.3.2 (i) 4.3.2 (i) For biosecurity material that has been transferred to or received from a non co-located AA site what are records available?
- Name and type of AA site
- Date of movement
- Import permit
- Description of goods transferred
- Quantity of goods transferred
-
4.3.2 (ii) For biosecurity material that has been transferred to or received from a non co-located AA site was a copy of the department entry number and import permit number provided?
-
4.3.2 (iii) For biosecurity material that has been transferred to or received from a non co-located AA site is a record from the receiving facility available stating their acceptance of the goods and confirmation of the goods safe arrival?
-
4.3.3 Are records retained for a minimum period of 24 months after release from biosecurity control or disposed of the goods?
-
4.3.4 Can all records be made available to the Department within two business days, upon request?
-
NOTE for the BIP: Failure to comply with the approval requirements or any violation of the Act may result in the approval of the AA being withdrawn or suspended and legal action prompted.
4.4 Traceability
-
4.4.1 Are record keeping procedures/systems, which ensure adequate controls and the necessary evidence to verify identifiable links to biosecurity goods, in place?
-
4.4.2 Are containers holding goods subject to biosecurity control easily identified?
-
4.4.3 Are goods subject to biosecurity control stored in an area that is securely locked when unattended? NOTE: Video surveillance, alarms or other security monitoring methods may also be used.
Section 5.0 Biosecurity Waste
5.1 Biosecurity Waste Management
-
5.1.1 Is biosecurity waste segregated from other waste?
-
5.1.2 When is biosecurity waste disposed of? During a work session, at the completion of a session of work or stored in waste containment storage? Is the storage space adequate NOTE: A work session ends if there is any break (lunch, snack break) during which all the persons conducting work subject to biosecurity control are absent from the biosecurity area.
- Disposed of during work session
- Disposed of at the completion of the session of work
- Stored in waste containment storage
-
5.1.2 (i) Is the container used to store biosecurity waste cleanable and impervious to the waste being contained, free of defects, vermin proof and able to be closed?
-
5.1.2 (ii) Is the waste protected from unauthorised access?
-
5.1.2 (iii) Is the container labelled 'Biosecurity Waste'?
-
5.1.2 (iv) Is the biosecurity waste disposed of within 21 days of being generated for non-perishable waste and 48 hours for perishable waste?
-
5.1.2 Is biosecurity waste treated in the BC1 facility?
-
5.1.2 (i) Where is the biosecurity waste treated?
-
5.1.2 (ii) Is the treatment area fully enclosed within walls, doors, windows, floors and ceilings?
-
5.1.2 (iii) Is the floor of the treatment area impermeable?
-
5.1.2 (iv) Are hand disinfection facilities available in the treatment area?
-
5.1.2 (iv) (i) Which Department approved hand disinfectant is available?
-
5.1.2 (iv) (ii) Is the hand disinfectant in date?
-
5.1.2 (v) Is a spill kit containing a Department approved disinfectant available in the treatment area?
-
5.1.2 (v) (i) Which Department approved disinfectant is available?
5.2 Treatment of Biosecurity Waste
-
5.2.1 Is all biosecurity waste or waste potentially contaminated with goods subject to biosecurity control decontaminated or disposed of by a Department approved method?
-
5.2.1 (i) Which Department approved method is used to treat biosecurity waste?
- Autoclave
- Other Department approved method
- Approved import permit conditions
-
5.2.1 (i) (i) Which other Department Approved methods are used to treat biosecurity waste?
5.3 Packing of Biosecurity Waste
-
5.3.1 Are small articles such as test tubes or bottles packed in open mesh baskets or in autoclave bags?
-
5.3.2 Are screw caps on containers loosened prior to loading in the autoclave?
-
5.3.3 Are empty containers placed on their side in the chamber?
-
5.3.4 If autoclave bags are used are they opened prior to loading, water added, slashed or tied with a melting tie?
-
5.3.4 (i) How are autoclave bags managed before loading in the steriliser?
5.4 Autoclave Parameters and Validation
-
5.4.1 Is the minimum continuous holding times after attainment of temperature 15 minutes at 121 degrees Celsius and 103kPa or 3 minutes at 134 degrees Celsius and 203kPa?
-
5.4.2 For each load are physical parameters met (i.e. the Department approved time and temperature is met in both the coolest part of the chamber and densest part of the load?
-
5.4.3 For every steriliser cycle is the temperature logged at one minute intervals or less or bacterial enzyme indicators used in both the coolest part of the steriliser and densest part of the load?
-
5.4.4 Does the sterilisation stage commence only when the correct temperature is reached in both the coolest part of the chamber and the densest part of the load?
-
5.4.5 Does the steriliser cycle used for the treatment of porous loads (i.e. lab coats) have a pre-vacuum stage?
-
5.4.6 Have the temperature gauges or sensors in the steriliser been calibrated to the temperature used for the treatment of bisoecurity waste?
-
5.4.7 Has the equipment used to calibrate the steriliser been calibrated and have a current certificate of calibration issued by a body with third-party accreditation (e.g. NATA)?
-
5.4.8 Is the sterilser calibrated at least every 12 months?
-
5.4.8 (i) When was the sterliser last calibrated?
-
5.4.9 Have biological lethality parameters been demonstrated each month through the use of biological indicators?
-
5.4.9 (i) Are the biological indicators placed in several positions in the load including the coolest part of the chamber and the densest part of the load?
-
5.4.9 (ii) Is a typical load included with the biological indicators?
5.5 Biosecurity Waste Records
-
5.5.1 Is a log book kept of all biosecurity that is generated and disposed in the facility?
-
5.5.1 (i) What information does the log book contain?
- Date
- User
- AA
- Biosecurity entry number
- Import Permit Number
- Cycle number
- Description of load
- if cycle successful
Section 6.0 Facility Pest Control
-
6.1 Is an effective pest control system in place to ensure that facilities are managed in a way that effectively isolates goods subject to biosecurity control from environments in which pest and disease are likely to become established?
-
6.1.2 Does the facility contain floor drains?
-
6.1.2 (i) Are the drain traps always filled with water and a suitable approved broad spectrum disinfectant (e.g. regular dosing program)?
-
6.1.2 (ii) Is solid waste collected from drains treated by an approved method?
-
6.1.2 (iii) Is a regular dosing program of the floor drains in place?
-
6.1.2 (iii) (i) Are records kept of the dosing regime, including the date, person and approved broad spectrum disinfectant?
-
6.1.2 (iv) Are the floor drains secure against entry by pests?
-
6.2 Are Pest Control treatment records readily available?
-
6.3 As a minimum, is a periodic inspection regime of the AA site in place?
-
6.3 (i) How often is the inspection undertaken?
-
6.3 (ii) Are records of the periodic inspections kept and easily available is the facility?
-
6.4 Is a knock-down spray (i.e. standard household aerosol insecticide spray) kept on-site at all times?
-
6.5 Is there a document (e.g. SOP) outlining all pest control measures available? NOTE: This document may include: the use of insecticides, fumigation, rodenticides, periodic inspections, baits and/or traps; a site plan with numbered bait stations; if applicable, contract details.
Section 7.0 Facility Operating Procedures
7.1 Spill Procedures
-
7.1.1 Is there a documented procedure in place to manage biosecurity related spills, including any spillage of goods subject to biosecurity, waste or waste water?
-
7.1.2 Is equipment used for the clean-up of biosecurity related spills provided within the AA site?
-
7.1.2 (i) What Department approved disinfectant is used in the spill kit?
- 1% Active sodium hypochlorite
- Virkon
- F10SC
- 80% (v/v) ethanol
-
7.1.2 (ii) What are the volumes of bleach and water used when making up the solution?
-
7.1.2 (iii) Based on the concentration of the starting of the sodium hypochlorite product are the correct volumes been used to gve a final concentration of 1% active sodium ?<br>Note: 20% solution required if product contains 5% active sodium hypochlorite, 10% solution required if product contains 10% active sodium hypochlorite
-
7.1.3 Does the documented biosecurity related spill procedure include:<br>- the equipment used for the clean-up of biosecurity related spills; and <br>- disinfectants to be used in the clean-up process?<br>NOTE: A list of broad-spectrum disinfectants can be found on the Department’s website.<br>
-
7.1.4 Is there a procedure in place to immediately report major spillage or loss of materials subject to biosecurity control to the Department?<br>NOTE: A major spillage is classified as a loss of material subject to biosecurity control outside the confines of the AA site, which cannot be readily cleaned up within 15 minutes, or which may be accessed by the general public.
7.2 Decontamination
-
7.2.1 Is equipment that comes into contact with goods subject to Biosecurity control cleaned and rendered safe by a Department approved method?<br>These include sterilisation, incineration and disinfection with an approved broad spectrum disinfectant.<br>
-
7.2.1 (i) How is the equipment decontaminated?
-
7.2.1 (i) (i) How is equipment decontaminated?
-
7.2.2 Are work surfaces immediately decontaminated with a department approved disinfectant on each occasion following work involving goods subject to biosecurity?
-
7.2.2 (ii) Which disinfectant is used to decontaminate work surfaces?
-
7.2.2 (iii) What quantities of water and sodium hyperchlorite are used to make the solution? NOTE: Check correct volumes are been used to give a !% active solution based on concentration of sodium hyperchlorite used.
-
7.2.3 Is there a hand wash basin fitted with hands free tap(s) or other means of decontaminating hands after handing goods subject to biosecurity control in the facility?<br>Note: Gloves must be removed and hands washed prior to leaving the premises<br>
-
7.2.3 (i) What facilities are available for decontaminating hands in the facility?
- Hand wash basin with hands free taps
- Dispenser fitted with an approved disnfectant
- Sink of hands free operation
- Sink with hand operated taps
- Other
-
7.2.3 (i) (i) What facilities are available in the facility to decontaminate hands?
-
7.2.3 (i) (i) Are hand basins located inside the facility, near the exit, and serviced with hot and cold potable water? NOTE: Alternatives to wash basins include:<br>- dispensers fitted with approved antiseptic solution (provided the dispensers can be operated without using hands);<br>- a sink of hands-free operation.<br>
-
7.2.3 (ii) What antiseptic hand wash is available in the facility?
-
7.2.3 (ii) (i) Please list the hand wash available?
-
7.2.3 (ii) (ii) Is the hand wash within its expiry date?
7.3 Personal Protective Equipment
-
7.3.1 When working with goods subject to biosecurity control, are personnel required to wear covering clothes (e.g. laboratory gown) and closed footwear?
-
7.3.1 (i) What PPE is required when working with biosecurity material?
- Front opening lab coat
- Rear fastening lab coat
- Disposable glooves
- Enclosed shoes
- Safety glasses
- Disposable lab coat
-
7.3.1 (ii) Is PPE segregated from unused PPE and PPE not used for work with biosecurity material?
-
7.3.1 (iii) When contamination occurs is PPE cleaned with a department approved disinfectant or laundered (commercially or non-commercially)?
-
7.3.1 (iii) (i) How often is PPE laundered?
- Weekly
- Fortnightly
- Monthly
- Quarterly
- Twice a year
- Once a year
- Never
-
7.3.1 (ii) Are the disposable lab coats and gloves disposed of as biosecurity waste?
-
7.3.2 Is a procedure in place which ensures gloves and protective garments are removed and hands are washed prior to leaving the AA?
-
7.3.3 Are food and beverage items for human consumption prohibited from being brought into the facility?
Section 8.0 Facility Management
8.1 Facility Maintenance and Cleanliness
-
8.1.1 Is the AA site managed in a way that ensures that the building and/or structures are maintained in a state of good repair?
-
8.1.2 Is the AA site kept in a clean state, free of a build-up of dust and dirt?
-
8.1.3 Are work surfaces finished with a material that is impermeable to liquids and joints sealed?
-
8.1.4 Is there a maintenance and cleaning schedule for the facility?
Section 9.0 Facility Isolation
-
All subsequent Sections and questions pertain to Biosecurity Containment Level 2 Facilities.
-
9.1 Are write-up areas constructed such that horizontal surfaces are minimised (e.g. minimal shelving)?
-
9.2 Are generic office functions prohibited from being undertaking at the write-up area within the AA?
-
9.3 Are the write-up areas provided within the AA used only to hold essential reference materials (e.g. technical equipment manuals)?
Section 10.0 Applicable Australian/New Zealand Standards - Indoor Animal BC2 Facilities
-
10.1 Is this an indoor animal facility?
-
NOTE for the BIP: The following standards from AS/NZS 2982.1 are the minimum for work with microorganisms at the BC2 level.
-
10.1.1 Where walls have a textured finish, are they easily cleanable and impermeable? NOTE: This may require: rendering, or coverage with plasterboard, of all brick work or blockwork, or filled and smoothing of mortar joints, and sealing of surfaces using a non-porous paint (e.g. elastomeric or latex paint).<br>
-
10.1.2 Where ceilings have a textured finish, are they easily cleanable and impermeable? NOTE: Acoustic tiles may be used for the ceiling where the facility does not perform a primary containment function, providing contaminants are not readily absorbed & can be removed easily by cleaning or washing. The ability for tiles to be deep cleaned through methods such as wash-down is desirable. <br>
-
10.1.3 Are safety showers or single use apparatus available in the facility?
-
10.1.3 (i) What clean up provisions are available?
- Plumbed Safety shower
- Single use packs
-
10.1.3 (i) (i) Are the single use packs within their expiry date?
-
10.1.3 (ii) Is the approach to all clean-up provisions unobstructed?
-
10.1.4 Does the facility contain either a handwash basin fitted with hands free taps or some other means of decontaminating hands?
-
10.1.4 (i) What means of decontaminating hands are available in the facility?
- Hand wash basin with hands free taps
- Dispenser fitted with an approved disnfectant
- Sink of hands free operation
- Sink with hand operated taps
- Other
-
10.1.4 (i) (i) Is an approved hand disinfectant available?
-
10.1.4 (i) (i) (i) Is the disinfectant within its expiry date?
-
10.1.4 (i) (i) Is the antiseptic hand pump hands free and approved by the Department?
-
10.1.4 (i) (ii) Is the antiseptic in date?
-
10.2 Is the facility a microbiology facility?
-
10.2.1 Where walls have a textured finish, are they easily cleanable and impermeable? NOTE: This may require: rendering, or coverage with plasterboard, of all brick work or blockwork, or filled and smoothing of mortar joints, and sealing of surfaces using a non-porous paint (e.g. elastomeric or latex paint).<br>
-
10.2.2 Are safety showers or single use apparatus available in the facility?
-
10.2.2 (i) What clean up provisions are available?
- Plumbed Safety shower
- Single use packs
-
10.2.2 (i) (i) Are the single use packs in date?
-
10.2.2 (ii) Is the approach to clean up provisions unobstructed?
-
10.2.3 What means of decontaminating hands are available in the facility?
- Hand wash basin with hands free taps
- Dispenser fitted with an approved disnfectant
- Sink of hands free operation
- Sink with hand operated taps
- Other
-
10.2.3 (i) Is a Department approved hand disinfectant available?
-
10.2.3 (i) Is the disinfectant in date?
-
10.2.3 (i) Is the antiseptic in date?
-
10.2.4 Are access doors to the facility fitted with self-closing devices?
-
10.2.5 Is the direction of air flow in the facility inward?
-
NOTE for the BIP: The following standards from AS/NZS 2243.3 are the minimum for work with indoor animal goods at the BC2 level.
Large HVAC heat exchangers (e.g. chilled beams and some other natural or forced convectors) located within containment occupancies may be vulnerable to contamination, if they are not protected by efficient air filters. The likely contamination and implications for containment should be carefully assessed where such devices are proposed or are used.
Mechanical ventilation should be provided to ensure that directional air flow is maintained
The primary air handling unit and fixed exhaust systems must be capable of maintaining the required directional air flow. Supplementary exhaust created by fume hoods or other special service exhaust systems will only be considered when determining compliance with ventilation requirements where the units are hard-wired and in constant operation or otherwise interlocked, to start and stop, with the supply air handling unit. A BC2 area may form part of a conforming PC2 area provided the air handler serving the BC2 area or combined BC2/PC2 areas has air filtration with performance rating not inferior to F4 to AS 1324. -
NOTE for the BIP: Where facility air is to be recirculated, filtration by a system with performance rating not inferior to F4 to AS1324 should be provided at the air handling unit intake at the facility bounding. Filter plenums and filters must be designed to capture and concentrate dust from the facility.
The use of ceiling plenum spaces as paths for the un-ducted re-circulation of air should be considered with caution. This practice can give rise to long-term build-up of settled laboratory dusts in ceiling spaces that may be disturbed if tiles are removed.
Where filtration is not provided at the air handling unit intake at the laboratory boundary, ducting must be installed between the intake and the return air discharge point. Prior to discharge at the return air discharge point, re-circulated air must be filtered at a point within the system.
Section 1
-
1.1 Is a sign showing the level of containment prominently displayed at each entry to the AA site where goods subject to biosecurity control are held?
-
1.1.1 Is the biosecurity sign permanently affixed and of professional standard?
-
1.1.2 Is the biosecurity sign made to state: ‘Biosecurity Area – Microbiological Facility - BC2 Facility - Authorised Persons Only, No Entry or Removal of Goods, Penalties Apply, (Biosecurity Act 2015)’; or as directed for specific biosecurity operations?
-
1.2 Is the name and telephone number of the Facility Manager or other responsible person[s] displayed near all access doors?
-
1.3 Are the doors to the site closed while biosecurity work is in progress to prevent unauthorised removal of goods subject to biosecurity control or the escape of pest and disease organisms?
-
1. 4 Is there a procedure/process in place to immediately notify the Department of any incidents which could significantly compromise the biosecurity security of the facility? NOTE: This may include structural damage, electrical breakdowns, escape or unauthorised entry and the removal of material subject to biosecurity control.<br>
-
1.5 Are measures in place (for example swipe card, door locks) to control access to the AA site?
-
1.5.1 Has the physical security system being verified in the last 12 months?
-
1.6 Is a current contingency plan in place to manage unexpected events that threaten to compromise biosecurity containment at the AA? (Unexpected events include appearance of pests, structural damage, unauthorised removal of biosecurity goods, spillage of goods subject to biosecurity control).
-
1.7.1 When was the contingency plan last reviewed?
Section 2
-
2.1 Have there been any structural and/or fitting changes to the AA site?
-
2.1.1 Have changes to the AA site been carried out in a manner which preserves consistency with: <br>- the third party certification; <br>- conformance to the AA requirements; <br>- the conditions of approval; and <br>- continues to comply with any subsequent amendments or revisions of AS.NZS 2982.1 & 2243.3?
-
2.1.2 Has a change significantly affected the overall containment system, including structural changes to 40% of the building? NOTE: A change that significantly affects the overall containment system requires re-certification. <br>
-
2.1.3 Has the Department been notified in writing within 15 working days of any alternations to the AA site management arrangements?
-
2.1.4 Where structural changes have been made to the AA site, has the Department been provided with a written statement describing the details of the alternations?
-
2.1.5 If requested, has the Department been provided with documented evidence of compliance with AS/NZS 2982.1 and 2243.3 when additions or modifications have been made to the facility?
Section 3
3.1 Biosecurity Area
-
3.1.1 Is a scale drawing, with dimensions and locations of biosecurity area(s) readily available? (Minimum A3 size)
-
3.1.1 (i) Is the location of containment devices, decontamination stations, PPE and treatment areas clearly indicated on the plans?
-
3.1.2 Is the biosecurity area of a size commensurate with the proposed quantity of goods being handled?
-
3.1.3 Are biosecurity areas and goods subject to biosecurity control accessible for the purpose of the inspection? NOTE: Accessible means goods must be able to be inspected as directed by a Biosecurity Officer. Generally, block staking will not be regarded as being accessible.
-
3.1.4 Are adequate controls and security measures in place for the biosecurity area?
-
NOTE for the BIP: Failure to comply with the approval requirements or any violation of the Act may result in the approval of the AA being withdrawn or suspended and legal action prompted.
3.2 Isolation
-
3.2.1 Are the biosecurity areas separate from other operations within the AA site?
-
3.2.1 (i) How are the biosecurity areas separated?
- Structurally
- Signage
- Distance
- Timing
- Other department approved method
- Lockable room
- Separate benches
-
3.2.2 Are cool/cold rooms, refrigerators, freezers or other storage units located outside the area were biosecurity work is undertaken? <br>NOTE: Where this is necessary, the AA site will need to have more than one biosecurity area.
-
3.2.2 (i) Is the additional biosecurity area located within the building that houses the facility? <br>NOTE: Movement procedures must be applied.
-
3.2.2 (ii) Is the additional biosecurity storage area (e.g. refrigerator, freezer) stated on the scale drawings?
-
3.2.2 (iii) Is there a transfer procedure in place to ensure the safe movement of goods subject to biosecurity control from the AA site to the storage area?
-
3.2.2 (iv) Is the additional biosecurity storage area a fully enclosable space contained within walls, doors, windows, floors and ceilings; and are doors and windows lockable and secure?
-
3.2.2 (v) Does the storage location contain a hands free decontamination station adjacent to the exit?
-
3.2.2 (v) (i) Is the disinfectant approved by the Department and in date?
-
3.2.2 (vi) Is a spill kit containing Department approved disinfectants available?
-
3.2.3 Is the AA managed to ensure that effective separation is maintained between released biosecurity goods, domestic goods and goods subject to biosecurity control?
-
3.2.4 Which Department approved method is used to differentiate domestic goods from biosecuirty goods?
- Sealed Containers
- Storage in designated cupboard
- Storage in designated fridge
- Other
- Storage in designated incubator
-
3.2.4 (i) (i) Please specify the "other" method:
-
3.2.5 To prevent cross-contamination while work is being undertaken, is there separation of work subject to biosecurity control from other work?
-
3.2.5 (i) How is biosecurity work separated from other work?
- Microchipping
- Permanent branding
- Tattooing
- A cage labelling system
- Other
- Separation by time
- Physical separation
- Other
-
3.2.6 Is there a documented procedure in place should cross-contamination occur? NOTE: Should cross-contamination occur, all goods shall be treated as goods subject to biosecurity control.
-
3.2.6.1 What procedure is in place in the event of cross-contamination occurring?
- All material treated as biosecurity
-
3.2.7 In addition to the Department’s requirements, do Import Permit conditions and inspection procedures apply for some commodities?
Section 4
4.1 Biosecurity Training
-
4.1.1 Is a list available containing the names of persons performing the following roles: (i) AA manager, (ii) AA contact person, (iii) AA accredited persons?
-
4.1.1 (i) When was the list last up dated?
-
4.1.1 (ii) Does the list contain a date of preparation and version number?
-
4.1.2 Have persons undertaking work with biosecurity goods undertaken the appropriate third party training?
-
4.1.3 Have all persons associated with AA sites and goods subject to biosecurity control been deemed as a ‘fit and proper person', and confirmed their status in writing?
4.2 Biosecurity Records
-
4.2.1 Is a manual listing procedures and documents applicable to biosecurity, including emergency and maintenance procedures available within the facility in a prominent position?
-
4.2.2 Are records for each consignment of goods subject to biosecurity control kept and maintained?
-
4.2.3 (i) What records are kept for each consignment of goods subject to biosecurity control?
- Current holding of goods subject to biosecurity control (Identified by scientific name)
- Date of arrival and total quantities
- Location or part of AA site where each item subject to biosecurity control is held
- Any direct or indirect derivatives
- Import Permit or Import permit number
- Date of completion of research
- Method & date of goods disposal/destruction (if applicable)
- The date & the Department’s permission for any movement (including transfer certificates) of goods from the facility
- Biosecurity entry number
- Other
- Country of origin
-
4.2.3 (i) (i) Please specify what other records are kept for biosecurity material:
-
4.2.6 Is a document detailing the annual UBC inspection of the facility available to the department for audit purposes? The minimum requirements for this document include:<br>• an annual inspection report detailing findings
-
4.2.11 Are records retained for a minimum period of 24 months after release from biosecurity control or disposed of the goods?
-
4.2.12 Can records be made available within 48 hours for inspection by the Department?<br>NOTE: The Department will continue to assess whether activities and arrangements have been implemented effectively and are meeting requirements. If records are unavailable during an inspection/audit, the Department will return to the AA site within 48 hours to continue the assessment of documents. This inspection will be charged at fee-for-service rates.
4.3 Traceability
-
4.3.1 is a system in place for all goods subject to biosecurity control which:<br>a) identifies and dates their arrival<br>b) Tracks the creation of direct/indirect derivatives<br>c) Enables clear reconciling of the goods to import permits, biosecurity directions and other documentation such as shipper's declarations, treatment
-
4.3.1 (i) Is the system maintained and kept up to date?
-
4.3.2 Is a identification system in place which enables biosecurity goods to be directly identified either:<br>a) with the scientific, and where identified or used, the common name, or<br>b) by a coded system, enabling reconciling of the goods to teh scientific name, and where identified or used the common name.
4.4 Transport
-
4.4.1 Is biosecurity material transported to a co-located facility?
-
4.4.1 (i) Is a transfer procedure for the safe movement of goods subject to biosecurity control between co-located facilities in place?
-
4.4.1 (ii) What details are included in the documented transfer procedure?
- Proposed suitable transport containers
- A map outlining the path on which the goods subject to biosecurity control is to be taken
- Other relevant information
-
4.4.1 (ii) (i) Please specify the "other relevant information"
-
4.4.1 (iii) Is the material package in a primary container that is sealed, shatter proof and crush resistant?
-
4.4.1 (iv) Is the outer container labelled with details of the contents and sender?
-
4.4.1 (v) What records are maintained?
- Name and type of AA site (forwarded to or received from) and containment level
- Date of movement
- Import permit or Import permit number
- Type of goods subject to biosecurity control (species/description)
- Total quantities (kg, litres) or total numbers.
-
4.4.2 Is biosecurity material transported to a non-co-located facility?
-
4.4.2 (i) What records are maintained?
- Approval number (forwarded to or received from), type classification and containment level)
- Date of movement
- Copy of biosecurity entry direction or direction number
- Import permit or import permit number
- Type of good subject to biosecurity control and total quantities
- Notification of acceptance from the receiving AA
Section 5
5.1 Disposal Site
-
5.1.1 Is the treatment room located outside the AA site?
-
5.1.1 (i) Is the treatment room lockable? (N.B. DAWE auditors will verify if doors are lockable)
-
5.1.1 (ii) Are floors, doors, walls and ceilings smooth and impermeable to liquids?
-
5.1.1 (iii) Is a hands free decontamination station available adjacent to the exit?
-
5.1.1 (iii) (i) Is the disinfectant approved by the Department and in date?
-
5.1.1 (iv) Is a spill kit containing Department approved disinfectants available in the treatment room?
-
5.1.1 (v) Is solid biosecurity wastes bagged and placed in an unbreakable container with a secured lid for movement within or outside the building to the approved disposal place?
-
5.1 Is there a document (e.g. SOP) in place that outlines how waste subject to biosecurity control will be effectively contained and rendered safe prior to disposal ? NOTE: Departmental approved methods of disposal of waste subject to biosecurity control include incineration at high temperature in a high efficiency incineration facility, deep burial, or sterilisation by autoclave.
5.2 Biosecurity Waste Holding Containers
-
5.2.1 Are holding containers for biosecurity waste awaiting sterilisation physically secure and protected from unauthorised access?
-
5.2.2 Segregated from other goods?
-
5.2.3 Cleanable and impervious to the waste being contained?
-
5.2.4 Labelled as 'Biosecurity Waste''?
-
5.2.5 Vermin proof and provide protection from loss, spread or spillage of biosecurity waste?
5.3 Biosecurity Waste Disposal
-
5.3.1 Is solid biosecurity waste disposed of by a Departmental approved method? NOTE: The Department’s approved methods of solid biosecurity waste disposal include: incineration at a high temperature in a high efficiency incineration facility, deep burial, or sterilisation by autoclaving.
-
5.3.1 (i) What method is used to dispose of solid biosecurity waste?
- Autoclave
- Deep burial
- Incineration
- Other approved method
-
5.3.1 (i) (i) Are the minimum autoclaving times after attainment of temperature for goods, residues or biosecurity waste: <br>- 121˚C (core temperature) for 15 minutes; or <br>- 121˚C for 30 minutes where core temperature is not measured?
-
5.3.1 (i)(ii) Have the temperature gauges and sensors been calibrated in the last 12 months?
-
5.3.1 (i) (ii) (i) Are NATA calibration certificates available for the equipment used to calibrate the autoclaves?
-
5.3.1 (i) (iii) Are the steriliser cycles validated by either: a) Cycle monitoring (Temperature/time) b) demonstration of lethality by indicators
- Cycle monitoring (time/temperature)
- Indicators
-
5.3.1 (i) (iii) (i) Is the time and temperature logged at 2 minute intervals (as a minimum)?
-
5.3.1 (i) (iii) (i) Are the indicators placed in the coolest and densest part of the load or in a single representative location for bench top autocaves?
-
5.3.1 (iii) Is a logbook (electronic or manual) recording steriliser load, temperature and duration of sterilisation cycle available?
-
5.3.1 (iii) (i) What information does the log contain?
- Date
- User
- Description of Load
- Import Permit Numer
- Biosecurity Entry Number
- AA Number
- If Cycle successful
-
5.3.1 (iv) Is the efficacy of the autoclave demonstrated by conducting spore tests?
-
5.3.1 (iv) (i) How often are spore tests carried out?
- Weekly
- Fortnightly
- Monthly
- Quarterly
- Twice a year
- Once a year
- Never
- Daily
-
5.3.2 Is all biosecurity waste water disposed of by a Departmental approved method? NOTE: Departmental approved methods include disposal of water by an approved municipal sewage system.<br>
-
5.3.3 Is all perishable biosecurity waste disposed of within 48 hours of being generated and non-perishable waste within 21 days?
-
5.3.4 Are sharps used with biosecurity material?
-
5.3.4 (i) Are the sharps containers labelled 'Biosecurity Waste'?
-
5.3.4 Does the facility have floor drains?
-
5.3.4 (i) Is solid waste collected from drains treated by an approved method?
-
5.3.4 (ii) Are the floor drains secure against entry by pests?
Section 6
-
6.1 Is there a document (e.g. SOP) outlining all pest control measures available? NOTE: This document may include: the use of insecticides, fumigation, rodenticides, periodic inspections, baits and/or traps; a site plan with numbered bait stations; if applicable, contract details.
-
6.2 Is an effective pest control system in place to ensure that facilities are managed in a way that effectively isolates goods subject to biosecurity control from environments in which pest and disease are likely to become established?
-
6.2.1 Are Pest Control treatment records readily available?
-
6.2.2 As a minimum, is a periodic inspection regime of the AA site in place?
-
6.2.2 (i) How often is the inspection undertaken?
- Weekly
- Fortnightly
- Monthly
- Quarterly
- Twice a year
- Once a year
- Never
- Daily
-
6.2.2 (ii) Are records of the periodic inspections kept and easily available?
-
6.2.4 Is a can of house hold pest knock down spray available in the facility at all times?
-
6.2.3 Is a documented procedure in place that ensures that the Department is notified of any pest or disease infestation?
Section 7
7.1 Spill Procedures
-
7.1.1 Is there a documented procedure in place to manage biosecurity related spills, including any spillage of goods subject to biosecurity, waste or waste water?
-
7.1.1.1 Does the document define major and minor spills?
-
7.1.2 Does the documented biosecurity related spill procedure include:<br>- the equipment used for the clean-up of biosecurity related spills; and <br>- disinfectants to be used in the clean-up process?<br>NOTE: A list of broad-spectrum disinfectants can be found on the Department’s website.<br>
-
7.1.3 Is equipment used for the clean-up of biosecurity related spills provided within the AA site?
-
7.1.3 (i) What Department approved disinfectant is used in the spill kit?
-
7.1.3 (i) Please list other disinfectant used:
-
7.1.3 (i) (i) What volumes of bleach and water are used to make the sodium hyperchlorite solution?
-
7.1.3 (i) (ii) Based on the concentration of the sodium hyperchlorite product are the correct volumes been used to achieve a final concentration of 1% active sodium hyperchlorite?
-
7.1.3 (i) (i) Is a concentration of at least 0.2% F10SC or a 1:500 dilution being used?
-
7.1.3.1 (ii) Does the disinfectant have a date of preparation or expiry?
-
7.1.4 Is there a procedure in place to immediately report major spillage or loss of materials subject to biosecurity control to the Department?<br>NOTE: A major spillage is classified as a loss of material subject to biosecurity control outside the confines of the AA site, which cannot be readily cleaned up within 15 minutes, or which may be accessed by the general public.
-
7.1.4 (i) What procedure is in place?
7.2 Decontamination
-
7.2.1 Is equipment, appliances, tools or utensils which have come into direct contact with goods subject to biosecurity control:<br>a) Decontaminated at the end of each work shift, or<br>b) placed in sealed, and appropriately labelled, containers for later reuse or decontamination?
-
7.2.1 (i) How is the equipment decontaminated?
- 80% (v/v) ethanol
- 1% Sodium hypochlorite
- Incineration
- Autoclave
- Other
-
7.2.1 (i) How is the equipment decontaminated?
-
7.2.2 Are paper towels or other disposable material used in cleaning associated with goods subject to biosecurity control or equipment which has been in direct contact with biosecurity material disposed of as biosecurity waste?
7.3 Personal Protective Equipment
-
7.3.1 Are suitable storage devices available that ensure separation of clean, used/reusable PPE?
-
7.3.2 What PPE is required when working with biosecurity material?
- Front Opening lab coat
- Rear fastening lab coat
- Disposable gloves
- Enclosed shoes
- Safety glasses
- Disposable lab coat
-
7.3.2 (i) Is dirty PPE laundered before re-use?
-
7.3.2 (i) (i) How often is PPE laundered?
- Weekly
- Fortnightly
- Monthly
- Quarterly
- Twice a year
- Once a year
- Never
- Daily
-
7.3.2 (i) (ii) Is PPE decontaminated before being laundered?
-
7.3.2 (i) (iii) How is PPE decontaminated?
-
7.3.2 (ii) (iv) Is a SOP available for the laundering of laboratory coats?
-
-
-
7.3.2 (i) Is the disposable laboratory coat disposed of as biosecurity waste?
-
7.3.3 Are gloves removed and hands thoroughly washed after handling goods subject to biosecurity control, and before leaving the facility? Used gloves shall be discarded with the biosecurity waste.
7.4 Containment Equipment
-
7.4.1 Is a biosafety cabinet used for any procedures with biosecurity material which may generate aerosols?
-
7.4.1 (i) Is a process in place for decontaminating the BSC after each work session?
-
7.4.1 (ii) Is paper towel used to decontaminate BSC disposed of as biosecurity waste?
-
7.4.1 (iii) Are the biosafety cabinets checked for containment efficiency and safety before initial use, after any modification after relocation and on an annual basis?
-
7.4.1 (iv) Are the biosafety cabinets certified annually be a qualified technician?
-
7.4.1 (iv) (i) Are the BSCs gaseous decontaminated prior to annual recertification?
-
7.4.1 (iv) (ii) What information does the report include?
- Model and date of test
- HEPA filter installation integrity
- Air velocity and uniformity in work zones
- Work zone integrity
- Containment at the aperture
-
7.4.1 (v) Does the BSCs contain vacuum pumps?
-
7.4.1 (v) (i) Are the 0.2 micron, hydrophobic vacuum filters changed every 3 years or when the filters show obvious contamination?
7.5 Facility Equipment
-
7.5.1 Is a centrifuge used with biosecurity material?
-
7.5.1 (i) Is the centrifuge fitted with sealed rotors or buckets?
-
7.5.2 Is a vacuum used with biosecurity material?
-
7.5.2 (i) Are the contents of the vacuum trapped treated as biosecurity waste?
-
7.5.1 (ii) Is the vacuum trap decontaminated with a department approved disinfectant?
Section 8
8.1 Facility Fixtures and Furnishings
-
8.1.1 Are fittings, fixtures (services), and furnishings within the AA site (for example, ceiling lights, air ducts, utility pipes, cable/conduit trays, furniture) smooth and cleanable?
-
8.1.2 Are all work surfaces cleanable, smooth, finished with a material that is impermeable to liquids, scratch resistant and have joints sealed? (DAWE auditors verify no obvious scratches, cuts, unsealed joints in work surfaces).
-
8.1.3 Are all under bench cupboards supported off the floor?
-
8.1.3 (i) Is the facility an existing AA?
-
8.1.3 (i) (i) Are the cupboards sealed to the floor?
8.2 Facility Maintenance and Cleanliness
-
8.2.1 Is the AA site managed in a way that ensures that the building and/or structures are maintained in a state of good repair?
-
8.2.2 Is the AA site kept in a clean state, free of a build-up of dust and dirt?
-
8.2.3 Does general cleaning of the facility only occur when no work is being undertaken on goods subject to biosecurity control?
-
8.2.4 Do service personnel only enter the facility to undertake maintenance/servicing activities when no work is being undertaken on goods subject to biosecurity control?
-
8.2.5 Does the facility have individually back flow prevention?
-
8.2.5 (i) Has the RPZD being tested within the last 12 months and are test reports available?
8.3 Facility First aid Provisions
-
8.3.1 Is a first aid cabinet/kit, which is fully stocked, readily accessible to persons working within the AA site?
-
8.3.1 (i) Are the contents of the first aid cabinet/kit within their expiry date?
Section 9
-
NOTE for the BIP: The AA site must comply with relevant safety codes and Work Health and Safety legislation.
-
9.1 Are write-up areas constructed such that horizontal surfaces are minimised (e.g. minimal shelving)?
-
9.2 Are generic office functions prohibited from being undertaking at the write-up area within the AA?
-
9.3 Are the write-up areas provided within the AA used only to hold essential reference materials (e.g. technical equipment manuals)?
Section 10
-
10.1 Where walls have a textured finish are they easily cleanable and impermeable?
-
10.2 Are safety showers or single use apparatus available in the facility?
-
10.2.1 What clean up provisions are available?
- Plumbed
- Single use packs
-
10.2.1 (i) Are the single use packs in date?
-
10.2.1 (ii) Are the safety showers tested as per the legislative requirements?
-
10.2.1 (iii) How often are the safety showers tested?
- Weekly
- Fortnightly
- Monthly
- Quarterly
- Twice a year
- Once a year
- Never
- Daily
-
10.2.2 Is the approach to clean up provisions unobstructed?
-
10.3 Does the facility contain either a handwash basin fitted with hands free taps or some other means of decontaminating hands?
-
10.3.1 What means of decontaminating hands are available in the facility?
-
10.3.1 (i) Can the sink be operated hands-free?
-
10.3.1 (ii) Is a hands-free dispenser containing an approved antiseptic solution located next to the handwash basin?
-
10.3.1 (iii) Is the antiseptic solution in date?
-
10.3.1 (iv) Are hand drying provisions available immediately adjacent to the hands-free decontamination station?
-
10.3.2 (i) Is the antiseptic pump hands free and approved by the Department?
-
10.3.2 (ii) Is the antiseptic in date?
-
10.4 Is the direction of air flow in the facility inward?
-
10.5 Are access doors to the facility fitted with self-closing devices?
Section 11
11.1 Specific Requirements for BC2 microbiological, animal and plant facilities
-
11.1 Are non BC2 areas adjacent to the BC facilities accessed by a route other than through the BC2 area (e.g. BC2 areas are not permitted to serve as thoroughfares to adjacent non BC2 areas?
11.2 Specific Requirements for BC2 animal and plant facilities
-
11.2.1 Is the facility an animal facility?
-
11.2.1 (i) Are arrangements in place to ensure that animals are monitored daily for symptoms of illness, parasitic infections, injury or abnormal behavior?
-
11.2.1 (ii) Are cages and/or racks labelled to indicate the identity and date of any inocula given?
-
11.2.1 (iii) For animals under biosecurity control are procedures in place to report unexpected animal mortalities or incidence of disease to the Department?
-
11.2.1 (iV) Is an up to date inventory of animals under biosecurity control available in the facility?
-
11.2.1 (v) Is a record of all procedures performed on animals under biosecurity control available in the facility?
-
11.2.1 (vi) Are any openings in the walls, ceilings or roof screened at the containment boundary with fine mesh screens having an aperture size small enough to prevent entry or egress of insects?<br>Note: Aperture size small enough to prevent entry or egress of insects will require a maximum aperture size of 0.25mm or 250 microns. Suitable material includes stainless steel mesh of 0.16 mm wire gauge (0.25mm aperture).
-
11.2.1 (vii) Are restraints available for the animals housed in the facility?
-
11.2.1 (viii) Are the exhaust filters on IVC cage system replaced when loaded to their design limit or every 5 years?
-
11.2.1 (ix) Are the IVC exhaust filters disposed of as biosecurity waste?
-
11.2.1 (x) Are carcasses disposed of immediately after death?
-
11.2.1 (x( (i) Are the carcasses stored at 4C and disposed of within 48 hours?
BC 5.3
3.1 Hygiene and Isolation
-
3.1 (A.1) : Is the biosecurity area(s) separate from other operations within the premises?<br>NOTE: Examples of how quarantine area separation can be defined in a particular class of premises include isolation form main thoroughfares with yellow lines, structural separation, a lockable room or building, a person proof security fence, separate benches or similar structures.
-
3.1 (A.2): How are the biosecurity areas separated?
-
3.1 (A.3): Is biosecurity material stored?
-
3.1 (A.3.1): Is quarantine material stored within the premise?<br>NOTE: Examples of how storage separation can be achieved in a particular class of premises include cupboards, cool rooms, refrigerators, and freezers.<br>
-
3.1 (A.3.2): Is quarantine material stored outside of the premises?<br>NOTE: Separate outside storage areas is not applicable to QC3 (QC3 facilities may only have the autoclave outside the immediate facility but within the building).<br>
-
3.1 (B.1): Do you separate quarantine goods from other goods that are stored within the facility?<br>NOTE: Effective separation of all goods can be achieved by: an impervious physical barrier; or other departmental approved methods. <br>
-
3.1 (B.1.1): How is/are quarantine goods separated from other non-quarantine goods within the premises?
- Cupboards
- Dewars (including straws in dewars
- Storage in separate rooms
- Freezers (including shelf within freezer)
- Sealed container
- Other (Please specify)
-
3.1 (B.1.2): Is the separation method approved by the Department of Agriculture?<br>NOTE: Examples of effective separation for some classes of premises include, but not limited to: sealed containers; storage in separate rooms. <br>
-
3.1 (C.1): Is the QAP managed in a way that ensures that all buildings and/or structures are maintained in a state of good repair?
-
3.1 (D.1) Is an effective pest control system in place? <br>NOTE: The operations of adjacent facilities must be considered when determining any additional pest control measures to be implemented. <br>
-
3.1 (D.1.1): Are associated pest control records of a periodic inspection regime available?
-
3.1 (D.1.2): Are Pest Control records readily available?
-
3.1 (D.1.3): Is a knock-down spray (i.e. standard household aerosol insecticide spray) kept on-site at all times?
-
3.1 (D.1.4): Is there a document (e.g. SOP) outlining all pest control measures available?
3.2 Biosecurity Area
-
3.2 (A): is the biosecurity area of a size commensurate with the quantity ( or proposed quantity) of the goods being handled?
-
3.2 (B): Are goods accessible for the purpose of the inspection?<br>NOTE: Accessible means goods must be able to be inspected as directed by an auditor/officer. <br>
3.3 Security
-
3.3 (A.1): Is an appropriate biosecurity sign displayed where goods subject to biosecurity are stored or handled?<br>NOTE: Cardboard and paper signs are not acceptable.<br><br><br>
-
3.3 (A.2): Are biosecurity signs on external structures a minimum 600 mm x 400 mm with lettering a minimum 25 mm height; and weatherproof and resistant to the elements?
-
3.3 (A.3): Are biosecurity signs within structures a minimum 295 mm x 210 mm with lettering a minimum 8 mm height?
-
3.3 (B.1): Are procedures in place to ensure that the QAP is only accessible by authorised persons?
-
3.3 (B.2): Is there a procedure/process in place to immediately notify the DAWR of any incidents which could significantly compromise the biological security of the facility?<br>NOTE: This may include structural damage, electrical breakdowns, escape or unauthorised entry and the removal of material subject to biosecurity control.<br>
-
3.3 (B.3): Are goods subject to biosecurity stored in an area that is/are securely locked when unattended?<br>NOTE: Video surveillance, alarms or other security monitoring methods may also be used. <br>
3.4 Operating Procedures
-
3.4 (A.1): Is there a Biosecurity Spill SOP available for the premises?
-
3.4 (A.1.1): Does the spills document include: the equipment used; where applicable the cleaning of this equipment and the spillage area with a Department of Agriculture approved broad-spectrum disinfectant?
-
3.4 (A.2): Is there a Spill Kit appropriate to manage biosecurity spills available in the premises?
-
3.4 (A.2.1): Does the contents of the Spill Kit reflect the details of the Spill SOP?
-
3.4 (A.2.2): Are the Spill Kit contents within their expiry date?
-
3.4 (A.2.3): Is a brief note on the Spill procedure kept in the spill kit? <br>NOTE: This is only a recommendation only.<br>
-
3.4 (B): Is there a procedure in place to immediately report major spillage or loss of materials subject to biosecurity control to the Department of Agriculture?<br>NOTE: A major spillage is classified as a loss of material subject to biosecurity control outside the confines of the QAP, which cannot be readily cleaned up within 15 minutes, or which may be accessed by the general public. <br>
-
3.4 (C): Is there a document detailing the entire imported goods pathway, which includes all the biosecurity operations?
-
3.4 (D): Is a procedure (e.g. SOP) in place which ensures that the Department of Agriculture is notified of any pest or disease infestation?
3.5 Administration and Management
3.5.1 Record Requirements
-
3.5.1 (A): Are electronic or manual records of all goods subject to biosecurity control imported through the QAP retained and available?<br>NOTE: This includes retaining originals or copies of Import Permits, biosecurity entries/directions or transfer approvals.<br>
-
3.5.1 (B): Are records retained for a minimum period of 18 months after biosecurity clearance or disposal of the goods?<br>NOTE: Records must be available within 48 hours of inspection by the Department of Agriculture.<br>
3.5.2 Office and General Premises Requirements
-
3.5.2 (A): Is a first aid kit/cabinet readily accessible to persons working within the premises?
-
3.5.2 (B): Are the contents of the First Aid Kit/cabinet within their expiry date?
3.5.3 Administration
-
3.5.3 (A.1): Is a scale drawing, with dimensions and locations of biosecurity area(s) readily available?
-
3.5.3 (A.2): Where appropriate, is/are direction or prior written approval to move, accept, transfer or release any goods subject to biosecurity control from the approved facility to another Department of Agriculture & Water Resources’ approved facility that is not co-located readily available?
-
3.5.3 (A.3): Where applicable, is a transfer procedure for the safe movement of goods subject to biosecurity control between co-located facilities?
-
3.4.2(A.3.1): Does the transfer procedure include a map outlining the path on which the biosecurity material is to be taken?
-
3.5.3 (B.1): Was the AA constructed so that it achieves upon commissioning an air leakage rate of no more than 120L/min when at a differential pressure of 200 Pa is applied?
-
3.5.3 (B.2): After commissioning, has the air leakage rate of the QAP been retested at least once every 3 years?
-
3.5.3 (B.2.1): When was the air leakage rate of the AA last retested?
-
3.5.3 (B.2.2): Has retesting demonstrated that the air leakage rate of the QAP is no more than 1200L/min maintained?
3.5.4 Management
-
3.5.4 (A): Is there a nominated responsible person[s] for the approved facility?
-
Nominated Person name:
Facility Type
-
Is the AA facility an Indoor Animal Facility?
5.1 General Requirements
-
5.1 (A): What work is conducted in the indoor animal containment facility?
- In vivo studies in animals using imported biological material.
- Other
-
5.1 (A.1): If Other, Please specify.
5.2 Hygiene and Isolation
-
5.2 (A): In the event of an unexpected death of an animal, are procedures in place to investigate the causes?
-
5.2 (B): Are post mortem examinations undertaken?
-
5.2 (B.1): Is there a separate area from other activities such as animal production provided to undertake post mortem examinations?
-
5.2 (B.2): Are adequate precautions taken to prevent cross-contamination where post mortem examinations are undertaken?
-
5.2 (C): Is secure housing/caging provided?
-
5.2 (D): Are write-up areas constructed such that horizontal surfaces are minimised (e.g. minimal shelving)?
-
5.2 (E): Are generic office functions prohibited from being undertaking at the write-up area within the AA?
-
5.2 (F): Are only essential reference materials (e.g. technical equipment manuals) held at the write-up area within the AA?
5.3 Waste Disposal
-
5.3 (A): Is there a document (e.g. SOP) in place that outlines how carcasses from animals subject to biosecurity control will be effectively contained and rendered safe prior to disposal?<br>Note: This should cover specific procedures for the disposal of any carcasses. <br>
-
5.3 (B): Is a procedure in place to accommodate situations where biosecurity carcasses cannot be disposed of immediately?
-
5.3 (C): Are biosecurity carcasses disposed of using an approved method?<br>Department of Agriculture approved methods of biosecurity carcass disposal include incineration at a high temperature in a high efficiency EPA approved incineration facility, deep burial, or sterilisation by autoclaving as per the Import Permit conditions. <br>
-
5.3 (D): Where applicable, are the minimum autoclaving times after attainment of temperature for all goods, residues or biosecurity waste: 121˚C (core temperature) for 15 minutes; or 121˚C for 30 minutes where the core temperature is not measured?<br>NOTE: Where the 15 minutes autoclaving time is used, the premises holder must specify how the core temperature has been reached and detail how this temperature was recorded. <br>
-
5.3 (E): Is animal bedding disposed of by method approved by the Department of Agriculture?<br>NOTE: Approved methods include, but are not limited to: incineration at a high temperature in a high efficiency EPA approved incineration facility, sterilisation or deep burial. <br><br>
-
5.3 (F): Is provision made for the decontamination of pens and cages? <br>NOTE: Decontamination can be achieved by: using a broad-spectrum disinfectant approved by the Department of Agriculture; or a method approved by the Department of Agriculture & Water Resources. <br>
-
5.3 (G): Is all biosecurity waste water disposed of by a method approved by the Department of Agriculture & Water Resources?<br>NOTE: Approved methods include disposal of waste water by an approved municipal sewage system. The use of other waste disposal methods must be approved in writing by the Department of Agriculture after demonstration of their efficacy to the satisfaction of the Department. Such methods may require detailed scientific research at the University’s expense. <br>
-
5.3 (H): Does solid biosecurity waste disposal include incineration at a high temperature in a high efficiency EPA approved incineration facility, deep burial or sterilisation by autoclaving?
-
5.3 (I): Where there are facility floor drains, are the drain traps always filled with water and a suitable approved broad spectrum disinfectant?
-
5.3 (J): Are floor drains secure against entry by pests?
5.4 Security
-
5.4 (A): Is a nominated staff member employed by Griffith University responsible for gate, door or animal enclosure keys or other access arrangements (e.g. swipe card)?
-
5.4 (A.1): Name of Staff Member Controlling Access to the Facility:
-
5.4 (B): Are doors closed when biosecurity work is in progress and/or when goods subject to biosecurity control are being stored in the facility?
-
5.4 (C): Is the name and telephone number of the facility manager or other responsible person[s] displayed near all access doors?
-
5.4 (D): Is a biosecurity sign displayed on the entry door to the facility?
-
5.4 (E): Is access to the facility for cleaning, servicing of equipment and repairs only occur after potentially contaminated surfaces have been disinfected with an approved broad-spectrum disinfectant?
5.5 Operational Procedures
-
5.5 (A): Do animals undergo in vivo trials?
-
5.5 (A.1): Are arrangements in place for animals undergoing in vivo trials involving imported biologicals to be checked daily?<br><br>
-
5.5 (A.2): Is a written record kept and available of daily checks of animals undergoing in vivo trails?
-
5.5 (B): Are animals subject to biosecurity control easily identifiable as biosecurity goods?<br>NOTE: Identification can be achieved by tattooing, microchip, permanent branding or cage labelling system<br>
-
5.5 (C): How are animals identified as biosecurity goods?
-
5.5 (C.1): If some Other method, please specify.
-
5.5 (D): Are cages and/or racks clearly labelled with the date and type of inoculation provided to the animals?
-
5.5 (E): Are animals subject to biosecurity control (alive or dead) transported from the containment facility?
-
5.5 (E.1): Is there a procedure that outlines the recording and decontamination of cages used to transport biosecurity animals?
-
5.5 (E.2): Are cages disinfected using a DAWR approved broad spectrum disinfectant or other approved method?
-
5.5 (F): Is there a procedure in place to report unexpected animal mortalities or incidents of disease?<br>NOTE: The procedure should include instructions regarding, but not limited to: The animal(s) being labelled with the day/date; where possible preserved in a refrigerator, cool room or freezer for post mortem by an Officer by the Department of Agriculture or a suitably qualified vet officer employed by the AA holder.<br>
-
5.5 (G): When working with goods subject to biosecurity control, are personnel required to wear covering clothes (e.g. laboratory gown) and closed footwear?
-
5.5 (H): Is dirty covering clothing removed and laundered before re-use?
-
5.5 (I): Do authorised users wash their hands after handling biosecurity animals?
-
5.5 (J): Where the facility has floor drains, are the drain traps filled with water and a suitable DAWR approved broad spectrum disinfectant?
-
5.5 (K): Does the premises have a Facility Manual containing emergency and maintenance procedures available in a prominent position?
5.6 Administration and Management
-
5.6 (A): Are records kept and maintained for each consignment of goods subject to biosecurity control?
-
5.6 (A.1): Do these records include:
- Biosecurity Entry Number (where relevant)
- Import Permit Number
- Description of the regulated goods (using accurate scientific terminology)
- Date of receipt of goods and country of origin
- Location or part of facility where each item subject to biosecurity control is held, and the respective BC status
- Records of any derivatives and additional cultures/material or substance grown from the original material subject to biosecurity control
- Where applicable, quantities (e.g. kg, litres) of goods received, destroyed and in storage
- Date of completion of research
- Details of any treatments
- Method and date of waste disposal/destruction
- The date of movement and the Department of Agriculture’s permission for any movement (including transfer certificates) of goods from the facility
- Comprehensive details of any breaches of goods subject to biosecurity control from the facility
-
5.6 (B): Is a record maintained of the current inventory of the animals present including a chronological record of procedures performed?
-
5.6 (C): Are records kept of births, mortality, post mortem findings, test results etc.?
-
5.6 (D): Can results of post mortem be made available on request?
-
5.6 (E): Are food and beverage items for human consumption prohibited from being brought into the facility?
-
5.6 (F): Is a procedure in place which ensures gloves and protective garments are removed and hands are washed prior to leaving the facility?
-
Is the AA facility a Microbiological Facility?
4.1 General
-
4.1 (A): What types of imported goods are held in the microbiological containment facility?
- Biological Samples
- Micro-organisms used for research
-
4.2 Hygiene and Isolation
-
4.2 (A): Are write-up areas constructed such that horizontal surfaces are minimised (e.g. minimal shelving)?
-
4.2 (B): Are generic office functions prohibited from being undertaking in the write-up area within the AA?
-
4.2 (C): Are only essential reference materials (e.g. technical equipment manuals) held in the write-up area within the AA?
4.3 Waste Disposal
-
4.3 (A): Where applicable, is biosecurity waste effectively contained and disposed of in a manner approved by the Department?
-
4.3 (B): Is there a document (e.g. SOP) outlining specific procedures for the disposal of any accumulated biosecurity waste?
-
4.3 (C): Where applicable, is solid biosecurity wastes bagged and placed in an unbreakable container with a secured lid for movement within or outside the building to the approved disposal place?
-
4.3 (D): Where waste cannot be disposed of immediately, is there as a minimum the provision for: NOTE: The separate storage device/area must be approved by the Department and be within the AA to prevent loss, spillage or unauthorised access.
- a separate storage device/area for the temporary holding of goods
- storage in lidded bins/containers of an appropriate size which are leak and pest proof
- bins to be labelled ‘Biosecurity Waste’
- double bagging of all waste
-
4.3 (E): Is solid biosecurity waste disposal by a method approved by the Department?
- Incineration at a high temperature in a high efficiency EPA approved incineration facility
- Sterilisation by autoclaving
-
4.3 (F): Where applicable, are the minimum autoclaving times after attainment of temperature for all goods, residues or biosecurity waste: 121˚C (core temperature) for 15 minutes; or 121˚C for 30 minutes where core temperature is not measured?<br>NOTE: Where the 15 minutes autoclaving time is used, the premises holder must specify how the core temperature has been reached and detail how this temperature was recorded.
-
4.3 (G): Where applicable, is all biosecurity waste water disposed of by an approved method?<br>NOTE: All biosecurity waste water disposal must also comply with requirements stated by: the state/territory EPA; local council; and the Water Board (if applicable).<br>
4.4 Security
-
4.4 (A): Is a logbook kept, recording visitor names, their company and the time and date of visits?
-
4.4 (B): Are doors closed when biosecurity work is in progress and/or when goods subject to biosecurity control are being held in the facility?
-
4.4 (C): Are the name and telephone number of the facility manager or other responsible person displayed near all access doors?
-
4.5 (D): Is a biosecurity sign displayed on the entry door to the facility?
-
4.6 (E): Does access to the facility for cleaning, servicing of equipment and repairs only take place after potentially contaminated surfaces have been disinfected with an approved broad-spectrum disinfectant?
4.5 Operational Procedures
-
4.5 (A.1): Are containers holding goods subject to biosecurity control clearly labelled using standard scientific nomenclature?
-
4.5 (A.2): Are containers holding goods subject to biosecurity control clearly labelled with the biosecurity entry number (where relevant); import permit number; and importation date? NOTE: If the containers cannot be labelled with this information due to constraints, such as size, then a suitable identification system may be used, such as referring to a logbook that contains the required information.
- Date of Importation
- Biosecurity Direction
- Import Permit Number
- Other
-
4.5 (A.2.1): If Other, please specify.
-
4.5 (B.1): Is equipment used or that has come into contact with goods subject to biosecurity control cleaned or rendered safe (decontaminated) by the Department by an approved method?
-
4.5 (B.2): How is equipment cleaned or decontaminated after use or before removal from the AA? Note: Approve methods include sterilisation, Autoclaving, swabbing with an approved broad spectrum disinfectant, incineration.
-
4.5 (B.3): Is equipment routinely disinfected?
-
4.5 (B.3.1): How is equipment disinfected?
-
4.5 (C): Are gloves removed and hands thoroughly washed after handling goods subject to biosecurity control, and before leaving the facility?
-
4.5 (D): Are used gloves discarded with the biosecurity waste?
-
4.5 (E): Is there separation of work subject to biosecurity control from other work to prevent cross-contamination?<br>Note: Physical or separation by timing?<br>
-
4.5 (F): When working with goods subject to biosecurity control, are personnel required to wear covering clothes (e.g. laboratory gown) and closed footwear?
-
4.5 (F.1): Is dirty covering clothing removed and laundered before re-use?
-
4.5 (F.2): Is a written procedure of how protective clothing will be laundered in place?
-
4.5 (G): Is the most recent internal annual inspection report detailing the findings available?
-
4.5 (H): Is a Facility Manual listing procedures and documents applicable to biosecurity, including emergency and maintenance procedures, available within the facility in a prominent position
-
4.5 (I): Is an autoclave used to decontaminate biosecurity waste and material?
-
4.5 (I.1): Is each steriliser (autoclave) cycle monitored?
-
4.5 (I.2): How is each steriliser (autoclave) cycle monitored?
- Thermocouples or resistance thermometers to ensure that the sterilisation temperature indicated has been achieved; or
- Chemical; indicators which progressively change colour with the time exposed at the specified temperature; or
- Biological indicators such as spore strips; or
- Enzyme indicators be used at regular intervals (e.g. monthly); or
- Other methods approved by the Department of Agriculture
-
4.5 (I.2.1): If Other, please specify.
-
4.5 (I.3): Where indicators are used, are they placed in several positions in a load, including those least likely to attain sterilization conditions?
-
4.5 (I.4): Is the annual checking and certification of sterilisers or heat ovens carried out by a qualified technician?
-
4.5 (J): Does the premise have a Biological Safety Cabinet?
-
4.5 (J.1): Are all biological safety cabinets used for biosecurity functions checked for containment efficiency and safety before initial use?
-
4.5 (J.2): Are all biological safety cabinets used for biosecurity functions checked after any modification including change of HEPA filters, after relocation and on an annual basis?
-
4.5 (J.3): Are used filters from biological safety cabinets disposed of with biosecurity waste?
-
4.5 (J.4): Where Class II Biological safety cabinets are used, have they passed the air barrier containment test in AS 1807.22?
4.6 Administration and Management
-
4.6 (A): Do records for each consignment of goods subject to biosecurity control include:
- Biosecurity Entry Number (where relevant)
- Import Permit Number
- Description of the regulated goods (using accurate scientific terminology)
- Date of receipt of goods and country of origin
- Location or part of facility where each item subject to biosecurity control is held, and the respective BC status
- Records of any derivatives and additional cultures/material or substance grown from the original material subject to biosecurity control
- Where applicable, quantities (e.g. kg, litres) of goods received, destroyed and in storage
- Date of completion of research
- Details of any treatments
- Method and date of waste disposal/destruction
- The date of movement and the Department of Agriculture’s permission for any movement (including transfer certificates) of goods from the facility
- Comprehensive details of any breaches of goods subject to biosecurity control from the facility
-
4.6 (B): Is a bi-annual summary of records, which includes all required information (e.g. as listed in question 4.6 (A)) readily available?
-
4.6 (C): Is a logbook (electric or manual) recording steriliser load, temperature and duration of sterilisation cycles maintained and available?
-
4.6 (D): Are current calibration certificates/records for all equipment that has a bearing on the biosecurity status of the material (e.g. autoclave) available?
-
4.6 (E): Are food and beverage items for human consumption prohibited from being brought into the facility?
-
4.6 (F): Is a procedure in place which ensures gloves and protective garments are removed and hands are washed prior to leaving the facility?
-
Is the AA facility a Plant Containment Level 3 (BC3) Laboratory?
6.1 General
-
6.1 (A): What work is conducted in the plant laboratory containment facility?
- In vitro use in imported seed samples;
- Plant material;
- Processed stock feed samples;
- Other
-
6.1 (A.1): If Other, please specify.
6.2 Hygiene and Isolation
-
6.2 (A): Are write-up areas constructed such that horizontal surfaces are minimised (e.g. minimal shelving)?
-
6.2 (B): Is generic office functions prohibited from being undertaking at the write-up area within the AA?
-
6.2 (C): Are only essential reference materials (e.g. technical equipment manuals) held at the write-up area within the AA?
6.3 Waste Disposal
-
6.3 (A): Is there a document (e.g. SOP) outlining how biosecurity waste will be effectively contained and rendered safe prior to disposal in a manner approved by the DAWR?
-
6.3 (B): Where applicable, is solid biosecurity waste bagged and placed in an unbreakable container with a secured lid for movement within or outside the building to the approved disposal place?
-
6.3 (C): Are procedures (e.g. SOP) where waste cannot be disposed of immediately?
-
6.3 (D): Where waste cannot be disposed of immediately, is there as a minimum the provision for:
- a separate storage device/area for the temporary holding of goods
- storage in lidded bins/containers of an appropriate size which are leak and pest proof
- bins to be labelled ‘Biosecurity Waste’
- double bagging of all waste
-
6.3 (E) Where applicable, is/are separate storage device/area within the AA, and/or approved in writing by the DAWR?
-
6.3 (F): Are storage devices and/or areas appropriately labelled?
-
6.3 (G): Is solid biosecurity waste disposed of by an approved method by the Department of Agriculture?<br>NOTE: Department of Agriculture approved methods of solid biosecurity waste disposal include incineration at a high temperature, in a high efficiency EPA approved incineration facility, deep burial or sterilisation by autoclaving.
-
6.3 (H): Where applicable, are the minimum autoclaving times after attainment of temperature for all goods, residues or biosecurity waste: 121˚C (core temperature) for 15 minutes; or 121˚C for 30 minutes where core temperature is not measured?<br>NOTE: Where the 15 minutes autoclaving time is used, the premises holder must specify how the core temperature has been reached and detail how this temperature was recorded.
-
6.3 (I): Where applicable, is all biosecurity waste water disposed of by an approved method?<br>NOTE: All biosecurity waste water disposal must also comply with requirements stated by: the state/territory EPA; local council; and the Water Board (if applicable). <br>
6.4 Security
-
6.4 (A): Are doors closed when biosecurity work is in progress and/or when goods subject to biosecurity control are being stored in the facility?
-
6.4 (B): Is the name and telephone number of the facility manager or other responsible person displayed near all access doors?
-
6.4 (C): Is a biosecurity sign displayed on the entry door to the facility?
6.5 Operational Procedures
-
6.5 (A): Is an annual inspection document readily available?
-
6.5 (A.1): Does the annual inspection document detail the findings, and the personnel who conducted the inspection?
-
6.5 (B): Is there documentary evidence that screens, filters and similar equipment have been cleaned in accordance with the manufacture’s specified frequency and procedures? <br>NOTE: This can be achieved by: supplying the frequency plan and procedures provided by the manufacturer; and/or recording the date that the cleaning occurred. <br>
-
6.5 (C): Is there a procedure (e.g. SOP) in place to report unexpected incidences of pest or disease to the Department of Agriculture immediately?
-
6.5 (D): When working with goods subject to biosecurity control, are personnel required to wear covering clothes (e.g. laboratory gown) and closed footwear?
-
6.5 (D.1): Are dirty covering clothing removed and laundered before re-use?
-
6.5 (D.2): Is a written procedure (e.g. SOP) in place that describes how protective clothing is laundered?
-
6.5 (E): Do authorised users wash their hands after handling plant material subject to biosecurity control?
-
6.5 (F): Where the facility has floor drains, are the drain traps filled with water and a suitable Department of Agriculture approved broad spectrum disinfectant?
-
6.5 (G): Is a Facility Manual listing procedures and documents applicable to biosecurity, including emergency and maintenance procedures, available within the facility in a prominent position?
-
6.5 (H): Is an autoclave used to decontaminate biosecurity waste and material?
-
6.5 (H.1): Is each steriliser (autoclave) cycle monitored?
-
6.5 (H.1.1): How is each steriliser (autoclave) cycle monitored?
- Thermocouples or resistance thermometers to ensure that the sterilisation temperature indicated has been achieved; or
- Chemical; indicators which progressively change colour with the time exposed at the specified temperature; or
- Biological indicators such as spore strips; or
- Enzyme indicators be used at regular intervals (e.g. monthly); or
- Other methods approved by the Department of Agriculture
-
6.5 (H.1.1.1): Where indicators are used, are they placed in several positions in a load, including those least likely to attain sterilization conditions?
-
6.5 (H.1.1.2): If Other, please specify.
-
6.5 (H.2): Is the annual checking and certification of sterilisers or heat ovens carried out by a qualified technician?
-
6.5 (I): Does the premises have a Biological Safety Cabinet?
-
6.5 (I.1): Are used filters from biological safety cabinets disposed of with biosecurity waste?
-
6.5 (I.2): Where Class II Biological safety cabinets are ued, have they passed the air barrier containment test in AS 1807.22?
6.6 Administration and Management
-
6.6 (A): Are records kept and maintained for each consignment of goods subject to biosecurity control?
-
6.6 (A.1): Do records for each consignment of goods subject to biosecurity control include:
- Date of receipt of goods
- Country or Origin
- Import Permit number, in vivo approval number or transfer approval plant
- Plant material type (where applicable include scientific name)
- Location or part of facility where each item subject to biosecurity control is held
- Date of completion of research
- Details of any treatment
- Method and date of disposal/destruction of of goods subject to biosecurity control (if applicable) and any direct or indirect derivatives
- The date of movement and the Department of Agriculture permission for any movement (including transfer certificates) of goods from the facility
- Comprehensive details of any breaches of goods subject to biosecurity control from the facility
-
6.6 (A.2): Is a record maintained of an up to date inventory of the plant material present including a chronological record of procedures performed?
-
6.6 (A.3): Where an autoclave is used, is a logbook (electronic or manual) recording steriliser load, temperature and duration of sterilisation cycle maintained and readily available?
-
6.6 (B): Are food and beverage items for human consumption prohibited from being brought into the facility?
-
6.6 (C): Is a procedure in place which ensures gloves and protective garments are removed and hands are washed prior to leaving the facility?
-
Go to next Section...
Chemical Hazards
-
Are there identified or potential Chemical Hazards normally present within the facility?
Security & Administration
-
Have all the appropriate licences and approvals been obtained by the users and/or managers?
-
Have all required Risks Assessments for the activities taking place in the facility been completed?
-
11.1.3 What online Induction Modules are required to be completed to access this facility?
- Emergency Response & Evacuation (Annual Fire Safety)
- Biosecurity
- Gas & Cylinder Safety
- General Biosafety
- Genetic Biosafety
- Health & Safety Induction
- Laboratory, Workshop & Studio Safety
- General Chemical Safety
- Animal Ethics
- General Radiation Safety
- Laser Safety
- Manual Tasks/Office Ergonomics
-
11.1.4 Have all the users completed the required online training?
-
11.1.5 Area site or facility inductions are required to be completed by users prior to gaining facility access?
-
11.1.5.A Please select the type of Facility or Site Induction that takes place
- Site Induction with Facility or Technical Manager for area.
- Site induction with academic supervisor or line manager.
- Online induction form.
- Other (please add response in comments)
-
11.1.6 Are records for inductions available and entered into the GSafe Certifications module?
-
11.1.7 Are staff and students aware of procedures in place for the use of high-risk chemicals (i.e. flammable, explosives, cytotoxins, poisons, hydrofluoric acid, carcinogens, etc.)?
-
11.1.8 Have materials that could easily be converted into cash or used for illegal purposes been identified and controls put in place to prevent theft?
-
11.1.9 Where workers or students are required to work after hours, are there controls in place to ensure their security?
-
11.1.10 Is the ban on eating, drinking and smoking in laboratories enforced?
-
11.1.11 Are personal possessions/student bags located out of access ways?
-
11.1.12 Are staff/students trained for the use of PPE used in their area?
-
11.1.13 Are staff/students familiar with spill clean-up requirements for their chemicals?
-
11.1.14 Are staff and students aware of procedures in place for the use of high-risk chemicals (i.e. flammable, explosives, cytotoxins, poisons, hydrofluoric acid, carcinogens, etc.)?
-
11.1.15 Is monitoring carried out where there is the possibility of exceeding chemical exposure limits?
-
11.1.16 Have the following PPE items been provided? (Consider correct selection, location, information & warning signs & maintenance);
- Coats/overalls
- Eye protection
- Gloves
- Safety Footwear
- Respirators etc.
- Self Contained Breathing Apparatus (Re-certified within last 12 months)
- Hearing Protection
- Helmets/Hard Hats
Spills Response
-
11.2.1 Are staff/students familiar with spill clean-up requirements for their chemicals?
-
11.2.2 Are there adequate spills response kits and equipment available within the facility, including for major spills?
-
11.2.3 Are the contents of the spills kits all present, within expiry dates and in sufficient quantities for the facility.
-
11.2.4 Are there Hydrofluoric Spills Kits required in the facility
-
11.2.4.A Is the location of the kit easily identified, easily accessible and located near to the area where Hydrofluoric Acid is handled?
-
11.2.4.B Does the kit contain Calcium Glucontate gel or similar for use in the event of contact exposure?
-
11.2.5 Where present, are safety showers and eye wash facilities functional, free from obstructions, and tested at regular intervals (weekly)?
Thermal Hazards
-
11.3 Are there identified or potential Thermal Hazards normally present within the facility?
-
11.3.1 Is all heating equipment clear of combustible materials?
-
11.3.2 Is all heating equipment used within the facility fit for purpose, well maintained and compliant with relevant design standards?
-
11.3.3 Are ovens, furnaces and kilns provided with flues?
-
11.3.4 Is suitable thermal protective equipment available for use with equipment; i.e. gloves, face shields, etc.?
-
11.3.5 Is welding/charring and similar activities conducted in a suitable workspace and clear of explosive/flammable/combustible materials?
-
11.3.6 Are open flames prohibited, especially in flammable and combustible liquids storage areas?
Ventilation & Containment
-
11.4 Are there fume cupboards present in the facility?
11.4 Fume Cupboards
-
11.4.1 Has the cupboard(s) been inspected and certified within the last 6 months?
-
11.4.2 Are restrictions posted near fume cupboards (< 2.5 L of flammables, no perchloric acid, etc.)?
-
11.4.3 Are volume restrictions observed?
-
11.4.4 Is the prohibition of storage of chemicals in fume cupboards enforced?
-
11.4.5 Are fume cupboards used safely?
-
11.4.6 Are Fume cupboards used for Perchloric and Hydrofluoric acids fitted with scrubbers interlocked to fan operation?
-
11.4.7 Are gases (LPG, O2, N2 etc.) supplied or used in fume hoods within the facility?
-
11.4.7 (i) List the gases supplied
-
11.4.7 (ii) Are they stored and handled appropriately?
Chemical Storage & Handling
-
11.5 Are chemicals handled or stored within the facility?
-
11.5.1 Is there secure/restricted access to Stores and/or storage areas?
-
11.5.2 Is the required chemical manifest present in Chemwatch and updated/maintained at least annually?
-
11.5.3 Do all staff at the lab have the required knowledge to access Chemwatch and obtain the correct Safety Data Sheet (SDS) for all chemicals within the laboratory?
-
11.5.4 Are all chemical stocks kept to a minimum (i.e. no excessive storage)?
-
11.5.5 Is there appropriate separation or segregation of incompatible classes/divisions of chemicals?
-
11.5.6 Are chemicals stored in completely separate storage rooms from gas cylinders?
-
11.5.7 Are storage surfaces covered with protective coatings or compatible with chemicals stored?
-
11.5.8 Are all storage devices (fridges, freezers) used for flammable liquid storage spark proof?
-
11.5.9 Are poisons, drugs, carcinogens, corrosives, etc. stored separately, as per endorsement conditions, and where necessary in locked cabinets?
-
11.5.10 Are chemical containers of the correct type?
-
11.5.11 Are all containers (including containers of decanted substances and stock solutions) correctly labelled?
-
11.5.12 Are containers of flammable and combustible liquids capped or covered when not in use?
-
11.5.13 Are containers in use of appropriate size e.g. <5L flammable liquids, <20L corrosives etc?
-
11.5.14 Are all containers on open shelves < 5L or 5kg in size?
-
11.5.15 On shelves over 1.5m high are chemicals in glass containers less than 1kg or 1L?
-
11.5.16 Are cabinets used for storage of large quantities of chemicals?
-
11.5.17 Are chemical storage cabinets used appropriately and are they maintained?auto closure of door(s) dedicated Dangerous Goods class storage, segregation of incompatible materials, and prohibition of combustible packaging?
-
11.5.18 Is the aggregate quantity of all chemicals in cabinets < 250L or or kg in a 10m radius?
-
11.5.19 Is there only one flammable liquid cabinet within a 10m radius (or only one 250L cabinet per 250 square metres) of floor space?
-
11.5.20 Is the separation distance between Flammable and Combustible Liquid cabinets >10m?
-
11.5.21 Are portable fire extinguishers available within 10m of storage location of flammable or combustible liquids?
-
11.5.22 Is flammable liquid storage well away from exits, and potential ignition sources?
-
11.5.23 Is bundling being used as a form of secondary containment ?
-
11.5.23 Are only approved pumps, drawing from the top of the storage containers, used to transfer flammable liquids?
Gases & Cylinders
-
11.6 Are gases stored or used in the facility?
-
11.6.1 Select the gases used/stored in this facility
- Acetylene
- Argon
- Argon (Liquid)
- CO2
- CO2 (Solid - Dry Ice)
- Hydrogen
- Helium
- Helium (Liquid)
- LPG
- Nitrogen (Compressed)
- Nitrogen (Liquid)
- Oxygen
- Specialty Mix
-
11.6.2 Has assessment for the potential for unsafe atmospheres, considering all gases stored in or supplied to the facility been undertaken?
-
11.6.3 Is there a gas monitoring system employing fixed and/or portable devices employed within the facility?
-
For all gas monitoring system non-compliances, lodge an incident report and contact the facility manager ASAP.
-
11.6.4 Is the equipment operating correctly at the time of the inspection?
-
Please provide photographic evidence of the non-compliance.
-
11.6.5 Are the sensor types and positions appropriate for the gases used in the facility
-
Please provide photographic evidence of the non-compliance.
-
Move to the next question.
-
11.6.6 Is equipment linked to an external alarm reporting system (e.g. BMS) or equipped with sirens/strobes visible external to the facility?
-
11.6.7 Have all components of the monitoring system undergone mandatory testing and calibration within the last 6 months?
-
Please provide photographic evidence of the non-compliance.
-
Move to the next question.
-
11.6.8 Enter the date of the most recent test.
-
11.6.9 Is equipment linked to an external alarm reporting system (e.g. BMS) or equipped with sirens/strobes visible external to the facility?
-
Go to the Next Question
-
11.6.10 Are flammable gas, oxidising gas and toxic gas cylinders segregated from each other or separated by a distance of at least three (3) metres?
-
Please provide photographic evidence of the non-compliance.
-
11.6.11 Are all cylinders stored vertically and individually restrained at 2/3 cylinder height?
-
Please provide photographic evidence of the non-compliance.
-
11.6.12 Where present, are gas cages and cylinder storage areas easily accessible, well maintained and clear of debris and flammable material?
-
Please provide photographic evidence of the non-compliance.
-
11.6.13 Are valves and regulators checked for integrity and tightness each time cylinders are exchanged?
-
11.6.14 Are flammable gas supply systems fitted with flashback arrestors?
-
11.6.15 Are cylinder trolleys readily available where cylinders are required to be moved or transported?
-
11.6.16 Are all staff undertaking cylinder exchange trained/inducted prior to being permitted to undertake the activity?
Manifest Quantity Workplaces
-
11.7 Is this a Manifest Quantity Workplace?
Placarding and Manifests
-
11.7.1 Are the outer warning “HAZARDOUS CHEMICALS” signs displayed and maintained at each site entrance?
-
11.7.2 Is placarding for tanks and storage areas of packages/drums displayed?
-
11.7.3 Has WHSQ been notified of the Manifest Quantity Workplace status?
-
11.7.4 Has the emergency plan been sent to QFES?
-
11.7.5 Is the manifest provided in a red weatherproof container at site entrances?
Fire and Explosion Risks
-
11.7.6 Are the extent of hazardous zones known in relation to flammable gases and liquids, e.g. 3m from FCL cabinets, 2m from flammable gas cylinders and a minimum of 3m from flammable liquid stores?
-
11.7.7 Are ignition sources controlled from entering hazardous zone(s)?
-
11.7.8 Are flammable or combustible materials no longer required by the workplace, including waste drums/containers etc., regularly removed off-site?
Spill Containment
-
11.7.9 Are Hazardous chemicals storage and handling areas provided with controls to contain spills within the site boundaries? They are maintained and inspected periodically?
-
11.7.10 Are spill compounds/bunds for any incompatible Hazardous chemicals independent from each other?
-
11.7.11 Are absorbent materials to soak up or respond to spilt liquids available and appropriate?
Impact Protection
-
11.7.12 Are containers and pipework for Hazardous chemicals protected from impact damage by mobile vehicles?
Fire Protection Systems
-
11.7.13 Is fire protection and firefighting systems at the workplace compatible with QFES’ equipment?
-
11.7.14 Does the workplace have an emergency plan that includes the Hazardous chemicals at the workplace?
-
11.7.15 Is all Hazardous chemical emergency equipment readily available and operational e.g. firehose reels, extinguishers?
-
11.7.16 What is the date of the last written record for the test/maintenance results for the fire protection and firefighting system components?
Use & Maintenance of Hazardous Chemical Controls
-
11.7.17 Are Hazardous Chemical controls inspected and maintained?
-
11.7.18 When are Hazardous Chemical controls reviewed?
-
11.7.18 "Other" was selected. Describe the Hazardous Chemical controls review process:
-
No. Go to next Section
Waste Disposal
-
11.8.1 Are there approved methods of disposal for all chemicals?
-
11.8.2 Are there sufficient and appropriate waste disposal containers? And bounded where required?
-
11.8.3 Are combustible waste materials and residues kept to a minimum, stored in appropriate containers and disposed of regularly?
-
11.8.4 Is excess waste regularly removed to the waste store and not accumulated in the work area?
-
Go to next Section...
Radiation Hazards
-
12. Are there identified or potential Radiation Hazards normally present within the facility?
-
12.1 Are there identified or potential Non-Ionising Radiation Hazards normally present within the facility?
-
Are Ultraviolet Radiation or Germicidal Lamps used in the space? 12.1.1 to 7
-
12.1.1 Are workers protected from exposure to UV equipment?
-
12.1.2 Have workers been trained in UV transilluminator safety?
-
12.1.3 Is appropriate eye protection available?
-
12.1.4 Are eye protection devices regularly checked for scratches, damage and colour change?
-
12.1.5 Do the laboratory gloves provide UV protection?
-
12.1.6 Are UV sources fitted with protective shields?
-
12.1.7 Are germicidal lamps interlocked to prevent exposure?
-
Are Nuclear Magnetic Resonance apparatus in use? 12.1.8 to 12
-
12.1.8 Is access to NMR magnets controlled?
-
12.1.9 Are appropriate warning signs posted?
-
12.1.10 Is the 0.5 mT line appropriately marked?
-
12.1.11 Are only (non magnetic) carbon dioxide fire extinguishers used in NMR areas?
-
12.1.12 Are only very experienced technicians allowed to fill the magnets with liquid cryogens?
-
Does Welding take place in the space? 12.1.13 to 15
-
12.1.13 Are arc-welding operators provided with helmets (with filter lens), fire resistant gauntlet gloves, apron, boots, spats, skullcap and boilermakers’ coverall or bib, brace & shirt?
-
12.1.14 Are eye protection devices regularly checked for scratches, damage and colour change?
-
12.1.15 Are welding screens available in the space?
-
Are lasers, other than those sealed in commercially available equipment, used in the space? 12.1.16 to 20
-
12.1.16 Is access to Class 3b or Class 4 lasers controlled?
-
12.1.17 Are appropriate laser safety procedures in place?
-
12.1.18 Are protective interlocks effective?
-
12.1.19 Are laser warning signs displayed?
-
12.1.20 Is eye protection provided?
-
12.2 Are there identified or potential Ionising Radiation Hazards normally present within the facility?
-
All Non and Partial Compliances noted in this section must be included in a report to the Possession Licensee, to be completed and submitted as soon as is practicable following the inspection.
-
12.2.1 Is there a Possession License required for sources present within this facility?
-
Please enter the information below as it relates to the Possession License.
-
12.2.2 Is the Possession Licence current?<br>
-
What is the expiry date?
-
When did it expire?
-
Please enter either Compliant or Non-Compliant only for this response...
-
Possession Licence Number:
-
Possession Licensee:
-
Nominee (If the Possession Licensee is not an individual person):
-
Approved Radiation Practice(s):
-
Do the authorities listed in the possession licence cover all the radiation practices conducted?
-
If there are conditions on the licence, have all these conditions been complied with?
-
Does each premises in use have a current and appropriate Certificate of Compliance displayed?
-
Please provide photographic evidence.
-
Please provide photographic evidence.
-
Is there a Local RSO appointed for the facility?
-
Enter the RSO/s First and Last names along with the expiry date of their certificate/s.
The RSO must either hold a valid RSO certificate, or be in one of the categories of person mentioned in Section 50 of the Radiation Safety Regulation. -
undefined
-
Select date
-
undefined
-
Select date
-
undefined
-
Select date
-
Do the authorities listed on the certificate/s cover the radiation practices conducted?
-
Is access to, or use of, radiation sources adequately controlled?
-
Has the RSO provided the training described in the RSPP?
-
Have all the persons who are required to participate in the training, done so?
-
Are all persons using a radiation source appropriately licensed or otherwise under the Act?<br>These person must either:<br> - hold a current an appropriate use licence, or <br> - be exempt under the Act.<br>(Also check for any conditions attached to use licences.)
-
Are all persons using a radiation source permitted by the possession licensee to use the source?<br>Check the RSPP for details about use of radiation sources.
-
Is the inventory of radiation sources correct?
-
Does each radiation source in use have a current and appropriate Certificate of Compliance affixed?
-
Was an Approval to Acquire obtained for each radiation source purchased in the past year?
-
Was an Approval to Relocate obtained for each radiation source permanently relocated outside of the Queensland in the past year?
-
If radiation sources have been disposed of, are there records of the disposal?
-
Can the approved RSPP be located?
-
Is the approved RSPP available to all persons using radiation sources?
-
Has the RSPP been reviewed within the past year or as described in the RSPP?
-
Have the results of any review been reported to the possession licensee?
-
Are personal monitoring devices provided to all persons who need to be monitored as described in the RSPP?
-
Are all monitored persons advised of the results of the dose assessment?
-
Is the personal monitoring record for each monitored person up-to-date?
-
Are personal alarm dosimeters supplied, checked, and used as described in the RSPP?
-
Are safety devices supplied, checked, and used as described in the RSPP?
-
Is the personal protective equipment supplied, checked, and used as described in the RSPP?
-
Is radiation monitoring equipment supplied, checked (or calibrated), and used as described in the RSPP?
-
Please provide photographic evidence of the equipment including certification labels/s.
-
Go to next Page...
6. Incident Reporting & Investigation
-
All WHS incidents involving workers, contractors, volunteers or visitors have been reported in GSafe.
-
For selected incidents, the following will be assessed:<br>• Action plan was developed <br>• Risk assessment was undertaken<br>• Risk controls were implemented and the incident closed in the system.
-
For notifiable incidents, the following will be assessed:<br>• The incident are notified to Work Health Safety Queensland via WHS Team.<br>• The incident was notified within 48 hours.
-
Significant first aid treatments is assessed to check if they have been reported.
7. Emergency Management
-
A workplace specific Emergency Management Plan has been developed following the Emergency Management guideline by the University.
-
A risk assessment has been completed as part of the Emergency Management Plan.
-
The Emergency Management Plan is reviewed annually and/or following an emergency or crisis and/or when improvement opportunities have been identified through emergency testing procedure rehearsals.
-
An emergency evacuation exercise has been conducted at least every 6 to 12 months.
-
Are occupants aware of Emergency Procedures and Emergency Contacts (e.g. Building Wardens, Floor Wardens, Security, and Health and Safety)?
-
Are Emergency Evacuation Diagrams available and are Emergency Exits clearly signed, adequately illuminated and unobstructed?
-
Extinguishers are in place, appropriate to fire-hazard and testing dates are current (as shown on tags).
-
Is fire response equipment inspected and tagged within the last 6 months, clearly signed and unobstructed?
8. First Aid
-
First aid requirements have been assessed for the school or workplace in consultation with the First Aid Officer and a First Aid Risk Assessment has been completed.
-
School or workplace complies with at least the minimum First Aid Officers, first aid kits and first aid rooms as described in the First Aid Risk Assessment Form.
-
First aid rooms (when applicable) are available and comply with the minimum first aid room requirements.
-
Regular inspections of first aid facilities, including a review of first aid kits are undertaken as planned or as required.
-
Infection control guidelines are understood by responsible person(s) (e.g. First Aid Officer).
-
First Aid Officer(s) has undertaken recognised first aid training that meets the requirements of:<br>• Provide First Aid - HLTAID003 <br>• Annual refresher for Cardiopulmonary Resuscitation (CPR) - HLTAID001.
-
Is the First Aid Kit clearly identified, accessible, maintained and adequate for the task?
9. WHS Induction and Training
-
An WHS induction process has been developed for workers that includes the training requirements specified in the WHS Induction Checklist. a training needs analysis is completed. Such as : <br><br>- Special equipment, <br>- Personal protective equipment, <br>- Safe work practices.<br>- Work health and safety legislation,<br>- Emergency procedures,<br>- First aid, <br>- Ergonomic and workstation assessment, <br>- Manual Handling, <br>- Use of plant and equipment, <br>- Working in Confined Spaces<br>- Working in isolation,<br>- Hazards and risks.<br>
-
WHS training has been scheduled and/or completed according to the WHS Training Plan/Register or equivalent template.
-
WHS Training Plan/Register or equivalent template has been developed and is kept up to date with additional training needs included where identified.
-
Based on job role, mandatory online WHS Program has been completed by workers.
10. Contractor Management
-
Does your work area engage in Contractor management
-
A Contractor Register or equivalent template is used to record approved contractors to work onsite.
-
An WHS induction process has been established for all contractors that includes the information specified in the Contractor WHS Induction requirements.
-
A review of contractor licences is undertaken to ensure they are relevant and valid, before commencement of of task.
-
All contractors report to the General Office upon arrival at the workplace, prior to commencing any works, and a visitor’s pass is issued.
-
A Safe Work Method Statement (SWMS) or equivalent is obtained from contractors prior to performing high risk activities :<br>• Hazardous manual handling<br>• Operating mobile and/or powered equipment<br>• Use of concrete<br>• Working at heights<br>• Use of hazardous substances and dangerous goods<br>• Working near electrical lines or systems.<br>• Hot Work<br>• Removal or disturbance of asbestos<br>
-
A Permit to Work system is established for contractors working in Confined Spaces (where applicable).
-
An WHS induction process has been established for volunteers, which includes the information specified in the Volunteer OHS Induction Checklist.
-
A risk assessment has been completed for volunteers prior to them undertaking
11. Purchasing
-
Goods that are purchased that have WHS implications are identified in the WHS Purchasing Checklist.
-
Hazards and risk controls measures for all purchases with WHS implications have been adequately assessed and documented in the WHS Purchasing Checklist.
12. Workers Compensation
-
A return to work coordinator (RTWC) who is a Principal, Assistant Principal or Business Manager has been defined. The designated Return to Work Coordinator (RTWC) has completed the required training
-
All claims tested have been adequately reported into GSafe.
-
All injured workers have been provided with key information
-
A workers’ compensation folder / file for each of the claims tested during the audit have been created including the minimum requirements in GSafe.
-
Return to work planning has been performed for all injured workers
-
There are clear processes in place to ensure Workers’ Compensation information is adequately stored and managed. Evidence is available to demonstrate that documents relating to injured workers’ claims are being sent to Workcover in a timely manner.
-
Is there awareness procedures for dealing with extended work hours and excessive job demands
-
Are support mechanisms for work-related stress, injury, illness or traumatic events known (i.e. Employee Assistance Program, Early Intervention Program, co-worker support)?