Title Page
-
Conducted on
-
Prepared by
-
Location
WT.01.01.01 Policies and Procedures
-
(EP1)The director named on the CLIA certificate approves a consistent approach for when waived test results can be used for diagnosis and treatment and when follow-up testing is required.
-
(EP2)The CLIA director/designee establishes written policies and procedures that address the following: - Clinical usage and limitations of the test methodology
-
(EP2)The CLIA director/designee establishes written policies and procedures that address the following: - Need for confirmatory testing (for example, recommendations made by the manufacturer for rapid tests) and result follow-up recommendations (for example, a recommendation to repeat the test when results are higher or lower than the reportable range of the test)
-
(EP2)The CLIA director/designee establishes written policies and procedures that address the following: - Specimen type, collection, and identification, and required labeling
-
(EP2)The CLIA director/designee establishes written policies and procedures that address the following: - Specimen preservation, if applicable
-
(EP2)The CLIA director/designee establishes written policies and procedures that address the following: - Instrument maintenance and function checks, such as calibration
-
(EP2)The CLIA director/designee establishes written policies and procedures that address the following: - Storage conditions for test components
-
(EP2)The CLIA director/designee establishes written policies and procedures that address the following: - Reagent use, including not using a reagent after its expiration date
-
(EP2)The CLIA director/designee establishes written policies and procedures that address the following: - Quality control (including frequency and type) and corrective action when quality control is unacceptable
-
(EP2)The CLIA director/designee establishes written policies and procedures that address the following: - Quality control (including frequency and type) and corrective action when quality control is unacceptable
-
(EP2)The CLIA director/designee establishes written policies and procedures that address the following: Test performance
-
(EP2)The CLIA director/designee establishes written policies and procedures that address the following: - Result reporting, including not reporting individual patient results unless quality control is acceptable
-
(EP3)If manufacturers’ manuals or package inserts are used as the policies or procedures for each waived test, they are enhanced to include specific operational policies (that is, detailed quality control protocols and any other institution-specific procedures regarding the test or instrument).
-
(EP4)The person from the hospital whose name appears on the Clinical Laboratory Improvement Amendments of 1988 (CLIA '88) certificate, or a qualified designee, approves in writing policies and procedures for waived testing at the following times:<br>- Before initial use of the test for patient testing<br>- Periodically thereafter, as defined by the person whose name appears on the CLIA certificate but at least once every three years<br>- When changes in procedures occur (for example, when manufacturers' updates to package inserts include procedural changes or when a different manufacturer is used)
WT.02.01.02 Responsible Staff Identified
-
(EP1) The person from the hospital whose name appears on the Clinical Laboratory Improvement Amendments of 1988 (CLIA '88) certificate, or a qualified designee, identifies, in writing, the staff responsible for performing waived testing.
-
(EP2) The person from the hospital whose name appears on the Clinical Laboratory Improvement Amendments of 1988 (CLIA '88) certificate, or a qualified designee, identifies, in writing, the staff responsible for supervising waived testing.
WT.03.01.01 Competency
-
(EP1)The person from the hospital whose name appears on the Clinical Laboratory Improvement Amendments of 1988 (CLIA '88) certificate, or a qualified designee, provides orientation and training to, and assesses the competency of, staff and licensed independent practitioners who perform waived testing.
-
(EP2) Staff and licensed independent practitioners who perform waived testing have received orientation in accordance with the hospital’s specific services. The orientation for waived testing is documented.
-
(EP3) Staff and licensed independent practitioners who perform waived testing have been trained for each test that they are authorized to perform. The training for each waived test is documented.
-
(EP4) Staff and licensed independent practitioners who perform waived testing that requires the use of an instrument have been trained on its use and maintenance. The training on the use and maintenance of an instrument for waived testing is documented.
-
(EP5) Competency for waived testing is assessed using at least two of the following methods per person per test:<br>- Performance of a test on a blind specimen<br>- Periodic observation of routine work by the supervisor or qualified designee<br>- Monitoring of each user's quality control performance<br>- Use of a written test specific to the test assessed
-
(EP6) Competence for waived testing is assessed according to hospital policy at defined intervals, but at least at the time of orientation and annually thereafter. This competency is documented.<br><br>Note 1: When a licensed independent practitioner performs waived testing that does not involve an instrument<br>and the test falls within his or her specialty, the hospital may use the medical staff credentialing and privileging<br>process to document evidence of training and competency in lieu of annual competency assessment.<br><br>Note 2: Provider-performed microscopy (PPM) procedures are not waived tests.
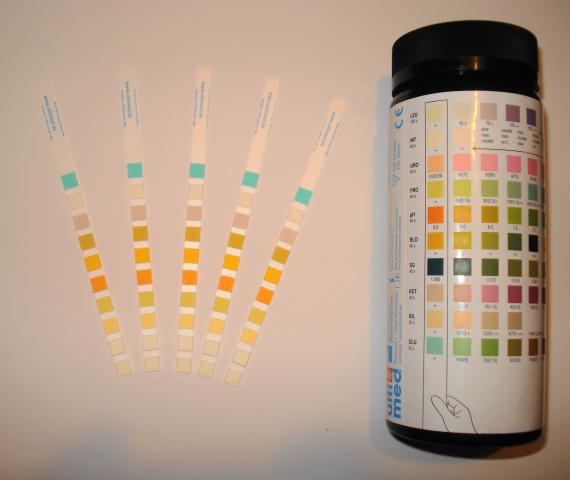
WT.04.01.01 Quality Controls
-
(EP1) The person from the hospital whose name appears on the Clinical Laboratory Improvement Amendments of 1988 (CLIA '88) certificate establishes a written quality control plan for waived testing that specifies the method (s) for controlling procedures for quality, establishes timetables, and explains the rationale for choice of procedures and timetables.
-
(EP2) The documented quality control rationale for waived testing is based on the following:<br>- How the test is used<br>- Reagent stability<br>- Manufacturers' recommendations<br>- The hospital's experience with the test<br>- Currently accepted guidelines
-
(EP3) For non-instrument-based waived testing, quality control checks are performed at the frequency and number of levels recommended by the manufacturer and as defined by the hospital’s policies.<br><br>Note: If these elements are not defined by the manufacturer, the hospital defines the frequency and number of levels for quality control.
-
(EP4) For instrument-based waived testing, quality control checks are performed on each instrument used for patient testing per manufacturers' instructions.
-
(EP5)For instrument-based waived testing, quality control checks require two levels of control, if commercially available.
WT.05.01.01 Waived Testing Records
-
(EP1) Quality control results, including internal and external controls for waived testing, are documented.<br><br>Note 1: Internal quality controls may include electronic, liquid, or control zone. External quality controls may include electronic or liquid.<br><br>Note 2: Quality control results may be located in the medical record.
-
(EP2) Test results for waived testing are documented in the patient's medical record.
-
(EP3) Quantitative test result reports in the medical record for waived testing are accompanied by reference intervals (normal values) specific to the test method used and the population served.<br><br>Note 1: Semiquantitative results, such as urine macroscopic and urine dipsticks, are not required to comply with this element of performance.<br><br>Note 2: If the reference intervals (normal values) are not documented on the same page as and adjacent to the waived test result, they must be located elsewhere within the permanent medical record. The result must have a notation directing the reader to the location of the reference intervals (normal values) in the medical record.
-
(EP4) Individual test results for waived testing are associated with quality control results and instrument records.<br><br>Note: A formal log is not required, but a functional audit trail is maintained that allows retrieval of individual test results and their association with quality control and instrument records.
-
(EP5) Quality control result records, test result records, and instrument records for waived testing are retained for at least two years.