Information
-
Audit reference number
-
Description of area/cell/process being audited
-
Site and Department
-
Scope of the audit:
-
Conducted by
-
Conducted on
-
Are there actions which require close out?
-
Revisit date (if there are actions to close out)
General
-
Take photographs and annotate them to help clarify any issues you identify as required in each section. Don't forget to record the good points as well.
You must select compliance level from the options:
OK = Satisfactory (It is working effectively and reliably)
NCR = Non-Conforming (It is NOT working effectively)
OBS = Observation (there may be a slight deviation but generally is working well)
OFI = Opportunity for Improvement (ideas that could make it better than it is)
N/A = Not Audited or Not Applicable (this question does not apply to the area being audited or it was not audited this time) -
Please ensure you write down, or photograph: serial numbers, reference numbers, order numbers etc to ensure that the audit is repeatable
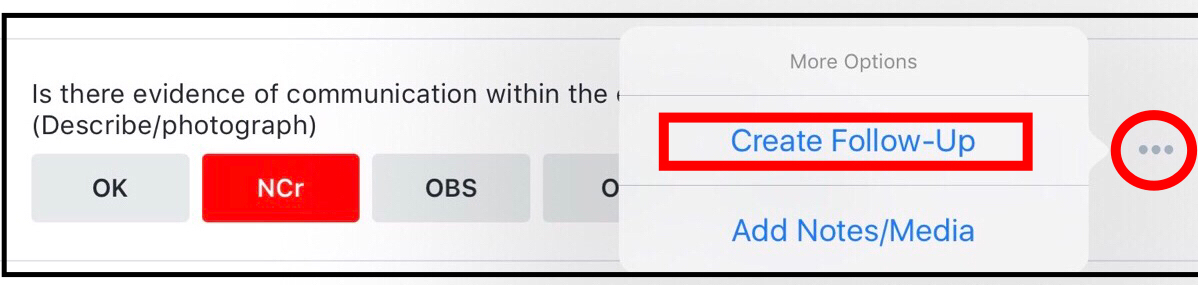
Add a follow up action against the question by selecting the three dots next to the question -
Photograph of area being audited
Checklist items
Additional Checklist items
-
Additional Check
-
Details of check performed (or process step)
-
Is this step compliant?<br>(Please add notes and photographs as needed)
-
Ensure that you complete a follow up action as necessary