-
Site conducted
-
Company
-
Date
-
Auditor
-
Location
-
This Checklist is based on the Code of Good Manufacturing Practice (Code of GMP) for the Feed Milling Industry (29/06/2009). It is designed for use by feed mills in assessing their level of compliance and for use by third party auditors to assess the feed mill during an audit. Auditors must use only this document as the most up-to-date checklist in conjunction with directions provided within the FeedSafe Certification Rules that define the Auditor’s responsibilities. FeedSafe certification is only for producers, there is no trading scope within this certification.
In Part 2 of this checklist a yes or no response should be provided for each question along with evidence in the observations and comments section. Where questions do not apply to the feed mill being audited, then a justification why it is not applicable must be written in the observations and comments section as well as n/a in the Yes/No column. The auditor may wish to note areas of improvement, such notes can be inserted within the ‘comments’ column.
Audit Direction Advice is provided to assist in the interpretation of some checklist questions and for reference to other guidance documents.
The mill being audited to the FeedSafe standard is required to declare to the auditor whether State Department audits have been completed since the last FeedSafe audit and the results of this audit. If the State Department found any area of non-compliance, including positive RAM test results for ruminant feeds, the FeedSafe auditor needs to place additional attention to these areas during the audit.
There are various guideline documents that are referred to in the Audit Checklist. Hyperlinks are provided in this electronic version. For the document linked from the SFMCA website resources, the reader needs a website access code. If you do not have your company’s access code, contact SFMCA at contact@sfmca.com.au.
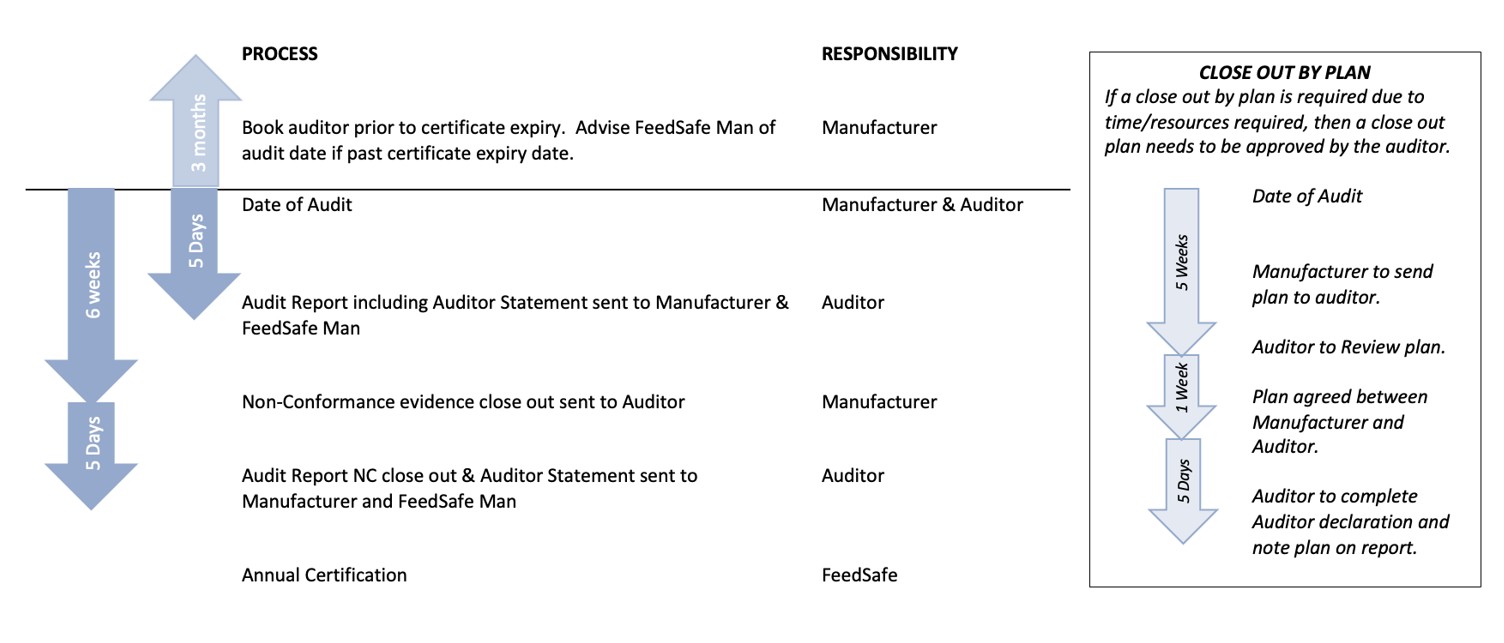
Part 3 of the checklist requires the listing of non-compliance areas, with a rating of major, moderate or minor. These are obtained by reviewing Part 2 questions which received a “No” response. This section of the checklist is also used to list the evidence sighted that address the non-compliance for close out.
Manufacturers are required to close-out the non-compliance areas by the due date, or FeedSafe Management may remove certification.
The final page of the Audit Checklist provides the Audit Statement that is required to be completed by the auditor and sent to the SFMCA within five calendar days of the conclusion of the audit, as well as the updated version upon close out of non-conformances within five calendar days of receiving evidence from the feed mill.
SFMCA issues FeedSafe certificates based on receipt of Audit Statements
PART 1 OVERVIEW & PREVIOUS AUDIT
Overview of Organisation
-
Details
Previous Non-Compliance Review
-
Audit
-
NC Number
-
Standard Reference
-
Previous non-conformance
-
Satisfactory
-
Comments
PART 2 AUDIT CHECKLIST
1 CERTIFICATION RULES
-
1.1 Is the site being audited a member of SFMCA?<br><br>-Proof can be a paid membership invoice.
-
1.2 Are the following current documents available by staff:<br>• the current version of FeedSafe® Standard - Australian Code of Good Manufacturing Practice for the Feed Manufacturing Industry (as amended or superseded)<br>• the current version of the FeedSafe® Certification Rules<br>• the National Biosecurity Manual for Feed Mills (as amended or superseded)<br>• the approved Manufacturing site’s quality system manual<br>• relevant safety data sheets for all materials stored on site<br><br>- All these documents must be available for staff to access. Show where they are kept.
-
1.3 Who is the QA Officer for the site?<br><br>-Include contact details of nominee.
-
1.4 What is the manufacturing tonnage for the past 12 months?<br><br>- Manufacturing tonnages can be substantiated in any way agreed to between the auditor and the auditee (e.g. production printouts) as long as proof is provided. A legitimate figure must be recorded. <br>This is a moderate NC if not provided.
-
1.5 Does the site have planning permission from the local shire?<br><br>-Provide documentation that proves shire permission has been granted.
2 GOOD MANUFACTURING PRINCIPLES
2.1 SITE
-
2.1.1 Is a site plan for the entire premises available?<br><br>-Site plan should identify major buildings, storage, processing areas and other features that impact on feed safety. Areas for storage of chemicals, medications and any hazardous goods should be shown on the site plan.
-
2.1.2 Does the site have suitable drainage?<br><br>-Reference should be made to poor drainage which presents a hazard to animal health and feed safety.
-
2.1.3 Are roadways maintained in good condition, dust and mud being minimised?<br><br>-Controls need to be in place to prevent contamination of feed with dust or mud. Site hygiene needs to include plans to upgrade areas immediately leading into intake and out-loading areas to prevent mud and dust contamination.
-
2.1.4 Can raw materials and finished feeds be unloaded and/or loaded without significant water damage resulting?<br><br>-Damage in terms of subsequent mould growth which may present a hazard to animals.
-
2.1.5 Is site security sufficient to ensure that accidental or deliberate contamination of product is avoided or prevented?<br><br>-Prevention of unauthorised site access with specific reference to access to chemicals and medications held on site. Attention should be given to security of receival intake pits and controlling people access to the site.
2.2 EQUIPMENT
-
2.2.1 Is appropriately designed and constructed equipment installed to meet the requirements of manufacturing stock feed?<br><br>-Emphasis on use of equipment designed for feed milling.
-
2.2.2 Is equipment in use designed and maintained to prevent contamination during the manufacturing process?<br><br>-Equipment should be in sound condition with minimal leaks of product. Confirmed through mill walk through looking for equipment leaks.
-
2.2.3 Is equipment designed and installed to allow for routine cleaning, maintenance and inspection?<br><br>-Relates to major pieces of plant and equipment such as hammer mill / roller mill, mixer, pellet press/cooler/crumble rolls, liquid additions, packing line. Confirm cleaning and maintenance practices through viewing records.
-
2.2.4 Is a preventative maintenance program in use?<br><br>- Confirm through viewing records for major pieces of plant and equipment.
-
2.2.5 Is there a system of logging maintenance work when completed?<br><br>- Confirm through viewing records for major pieces of plant and equipment.
-
2.2.6 Are monitoring and/or controlling devices (weigh scales, temperature probes, flow meters, etc) monitored for accuracy and recalibrated as per maintenance plan?<br><br>-A procedure for monitoring should define the method, frequency of checking and include the use of certified weights or a third-party operator where required with specific emphasis on critical control points.<br>Confirm through sighting records, e.g. certificates of calibration for weighbridges and trade scales as well as internal monitoring.
-
2.2.7 Are records kept of calibration monitoring?<br><br>-A procedure for monitoring should define the method, frequency of checking and include the use of certified weights or a third-party operator where required with specific emphasis on critical control points.<br>Confirm through sighting records, e.g. certificates of calibration for weighbridges and trade scales as well as internal monitoring.
2.3 STORAGE
-
2.3.1 Are storage areas designed and maintained to prevent damage to, contamination, unintended mixing, or spoilage of ingredients and packaging materials?
-
2.3.2 Are storage bins, silos, tanks and storage areas clearly identified with labels or numbers?<br><br>-These should match the site plan as per 2.1.1.
-
2.3.3 Are there written documentation of contents within storage facilities?<br><br>-The written documentation can be on a silo layout sheet, silo chart, whiteboard or computer program system.
-
2.3.4 Are storage silos, bins, tanks and sheds adequately designed, cleaned and maintained so that finished product quality is not compromised?<br><br>-Refer to the SFMCA document Feed Mill Hygiene Guide and FeedSafe Mill Hygiene Training Module.
-
2.3.5 Is there an inspection and maintenance program for storage silos, bins, tanks and sheds which prevents raw material or finished product quality being compromised?<br><br>-Silos and storage areas are checked either during stock take, preventative maintenance, or some other defined event. Confirm through sighting records.
-
2.3.6 Is a documented first in first out stock rotation in practice?<br><br>-FIFO (first in first out), or FEFO (first expired first out).
-
2.3.7 Are all packaged raw materials stored adequately, allowing separation of different raw materials?<br><br>-Reference to higher risk raw materials as identified in HACCP risk assessment (5.2).
-
2.3.8 Are bagged finished products stored in a manner that does not cause product damage and enables clear identification?
2.4 VENTILATION
-
2.4.1 Are ventilation or dust extraction units adequate to prevent accumulation within mill buildings of steam, dust and other airborne contaminants?<br><br>-Assessed through site walk through and demonstration of no accumulation of dust or condensation on mill walls, bins and equipment.
-
2.4.2 Is appropriate dust extraction equipment installed?<br><br>-Evidenced by no significant build-up of dust within mill buildings.
2.5 WASTE MANAGEMENT
-
2.5.1 Is waste and contaminated material controlled and regularly removed from the site?
-
2.5.2 Are waste containers clearly identified and maintained to ensure waste material is contained and not incorrectly used?<br>Where bulk or bag material is held for waste disposal, is it adequately labelled to ensure it is not incorrectly used?
2.6 CROSS CONTAMINATION CONTROL
-
2.6.1 Is there a written procedure adopted to prevent cross contamination of feeds with incompatible feed ingredients and medications?<br><br>-These need to be validated, refer to 8.1.
-
2.6.2 Are precautions taken to prevent cross contamination of subsequent mixes; this may include records of flushing, sequencing and cleaning?<br><br>-Evidence of documented records such as production sheets.
2.7 CLEANING
-
2.7.1 Is there a written mill cleaning procedure and schedule?
-
2.7.2 Are the buildings, grounds and machinery cleaned regularly?<br><br>-Seen through the site being in a clean and tidy condition. Need to verify based on mill cleaning records that this is an ongoing standard not just prior to audit.
-
2.7.3 Is there a system to verify the adequacy of the mill hygiene program?<br><br>-Need for documented evidence that the mill is cleaned regularly and that the mill has staff assigned to cleaning. Refer to the SFMCA document Feed Mill Hygiene Guide and FeedSafe Mill Hygiene Training Module, this includes a section on verifying hygiene.
2.8 PEST CONTROL
-
2.8.1 Does the site have a written pest control management program?<br><br>-Need to produce documented evidence that there are regular pest control management steps in place for pests of concern (eg. rodents, birds, insects).
-
2.8.2 Are storage areas clean and tidy and have steps been taken to minimise vermin and bird presence?<br><br>-Refer to the SFMCA document Feed Mill Hygiene Guide and FeedSafe Mill Hygiene Training Module
2.9 MEDICATIONS & CHEMICALS
-
2.9.1 Are feed additives and medications clearly identified and stored in accordance with labels and regulations?
-
2.9.2 Is this area adequately secure to prevent cross contamination or inappropriate handling?
-
2.9.3 Are S4 medications kept in a locked secure area?<br><br>-May be unlocked during working hours, however there needs to be demonstrated security controls for outside working hours. S4 use remains subject to relevant State licence control conditions.
-
2.9.4 Are veterinary chemical products in use registered by the APVMA?<br><br>-This can be confirmed through matching products to APVMA PubCRIS data.
-
2.9.5 Are veterinary chemical products used according to label instructions or veterinary prescription?
-
2.9.6 Are veterinary chemical instructions (prescriptions) provided by veterinarians kept on record?
-
2.9.7 Are all non-ingredient materials managed to ensure they are not mistakenly incorporated into stockfeed?<br><br>-Emphasis placed on chemicals which are either toxic to livestock or may result in chemical residues if unintentionally included within stock feed.
-
2.9.8 Is there a written inventory control system for all non-raw material chemicals used on site, including cleaning chemicals?<br><br>-Inventory control is for all chemicals used within or located at the feed mill. Examples are grain treatment chemicals and rodent control. Where other non-feed milling activities take place on the site and these are physically separated from the feed milling operations, they are outside the scope of FeedSafe e.g. chemicals used in vehicle maintenance are not included in the inventory where they are stored and used in buildings separate from the feed mill. Inventory control does not relate to lunchrooms, amenities or other non-feed milling buildings.
-
2.9.9 Are chemical treatments (e.g. fumigants, pesticides) applied as per label instructions to stored raw materials?<br><br>-There needs to be a system that records chemical treatment use. Verify through record inspection.
-
2.9.10 Are hazardous materials such as baits for pest control, boiler water treatment, fuel and cleaning agents stored securely away from ingredient handling areas to ensure that mistaken use in feed does not occur?<br>Where relevant are they stored close to the point of intended use?<br><br>-Hazardous materials need to be assessed with respect to safe storage location. E.g. baits ideally are stored away from the milling area; boiler chemicals are stored within the boiler area. This needs to be identified in the HACCP risk assessment.
2.10 RAM
-
2.10.1 Where the mill manufactures ruminant feeds, are separate receival hoppers available for handling Restricted Animal Material (RAM)?
-
2.10.2 If there is not a separate receiving hopper for RAM, are written procedures in place and followed to prevent cross contamination of non-RAM raw materials being received?
-
2.10.3 Are these procedures verified through inspection, sampling and testing?<br><br>-The auditor needs to sight the verification records as well as validations as per clause 8.1.
-
2.10.4 Is RAM stored in designated bins or storage areas?<br><br>-Importance relates to feed mills manufacturing ruminant feeds and storing or using RAM on site.
-
2.10.5 If unlabelled bagged restricted animal material is purchased, is such material either relabelled prior to storing on site or rejected and returned to the supplier?<br><br>-Ensure bulk RAM, which is rebagged, or bags with missing labels, are correctly labelled.
-
2.10.6 In mills where restricted animal material is used and ruminant feed is also manufactured, is there a system to identify formulations contain restricted animal material and is unsuitable for ruminant feeding?<br><br>-Confirm that the identification is recognised by manufacturing staff producing feed.
-
2.10.7 Are reworks and returns containing RAM or assumed to contain RAM clearly identified as such and are only reprocessed into non-ruminant feeds?
3 PERSONNEL & TRAINING
3.1 JOB DESCRIPTIONS & ORGANISATION CHART
-
3.1.1 Are qualified and/or experienced persons directly responsible on site for manufacturing operations?<br><br>-Identified through educational qualifications and/or industry experience in feed milling. An example of acceptable training is the SFMCA Advanced Feed Milling Course.
-
3.1.2 Are employees provided with written duties?<br><br>-These written duties can be in the form of job description, work procedure and/or work instructions. This is more than an office-based set of work instructions and needs to be operational within the mill. Employee written duties need to be linked to the feed safety assessment and critical control point integration through the manufacturing process.
-
3.1.3 Are relevant mill staff aware of the requirement to allow access to state authorities to obtain samples for auditing of the BSE ruminant feed ban?<br><br>-Results from any authority sampling and testing should be provided to the auditor.
3.2 TRAINING
-
3.2.1 Are employees trained in GMP as it relates to their duties?<br><br>-Refer to SFMCA FeedSafe Overview Training unit or equivalent GMP training.
-
3.2.2 Is completed training (including GMP training) documented in employee records?
-
3.2.3 Is the person who performs the on-site functions of production manager/supervisor appropriately trained?<br><br>-Either through industry training qualification (refer SFMCA Advanced Feed Mill Training Course) and/or work experience supported through on-site training. They need to be competent to perform the duties required.
-
3.2.4 Is there a training program and are staff adequately trained to competently carry out their assigned tasks?<br><br>-This includes provision to employees’ relevant written procedures and on the job training with an experienced operator. Refer to the SFMCA Advanced Feed Mill Training Course where relevant.
-
3.2.5 Does training encompass actions impacting on product safety, quality and the environment?<br><br>-This includes provision to employees’ relevant written procedures and on the job training with an experienced operator. Refer to the SFMCA Advanced Feed Mill Training Course where relevant.
-
3.2.6 Is there specific training related to the ruminant feeding ban including storage, handling and use of restricted animal materials?<br><br>-Only relevant where RAM is used on site. Refer to clause 2.10.
-
3.2.7 Are the personnel authorised to accept or reject raw material deliveries trained?<br><br>-Need to identify who is authorised to accept or reject raw materials outside specification.
-
3.2.8 If samples are tested on site, are staff responsible appropriately trained and equipped?<br><br>-Staff are required to be competent in sampling and testing and a finished product testing procedure would assist this process.
-
3.2.9 Are the personnel who apply chemicals, including pest control chemicals, trained and experienced (or licensed) in their use?<br><br>-Identify in training records for staff or service supplier advice.
-
3.2.10 Do appropriately trained personnel carry out maintenance and calibration of equipment?<br>Are maintenance staff trained to identify equipment faults which impact on product quality and safety?<br><br>-Either by external contractors or experienced operators. <br>Recognition of staff experience and knowledge of the site as well as training. Specific reference to faulty equipment resulting in cross contamination.
3.3 HYGIENE
-
3.3.1 Are personnel aware of their responsibilities and impacts to maintaining a hygienic environment?<br><br>Refer to clause 2.7 cleaning.<br>Refer to clause 3.2.5 training.
3.4 VISITORS & CONTRACTORS
-
3.4.1 Are there written procedures controlling both visitors and contractors entering the site?
-
3.4.2 Is there a written procedure to make all site visitors aware of their potential impact on product safety, quality and the environment?<br><br>-There needs to be documented steps taken to ensure visitor awareness.
4 DOCUMENT CONTROL
4.1 FORMULATIONS
-
4.1.1 Is there a written formulation master file, with a record of the dates of use and version numbers?<br><br>-Either in hard copy or electronic form.
-
4.1.2 Is this master file maintained by an authorised person?<br><br>-Confirm who is on the authorised person list and their experience or qualifications.
-
4.1.3 Do formulas in use provide the following information?<br> the name and unique identity code of the product.<br> an indication as to the animal type for which the product is intended to be fed.<br> the precise quantity of each raw material and, where appropriate, the location of the bin or bags of that raw material?
-
4.1.4 When formulations are modified, including raw material substitutions, does an authorised person make such modifications?<br><br>-Confirm who is authorised and their experience or qualifications.
-
4.1.5 Is there a system to document formulation changes when they are made?<br><br>-Records should be retained for at least twelve months (refer to clause 4.2.1).
-
4.1.6 Is there a documented procedure for treatment of returns and reformulation into feed?<br><br>-Confirm who is authorised and their experience or qualifications.<br>See also clause 8.4 Reworks.
4.2 RECORDS
-
4.2.1 Are production and batching records kept and retained for at least twelve months?<br><br>-Longer time periods for medication use records may be required in some States.
-
4.2.2 Are records kept allowing finished product trace back for a period of at least twelve months?<br>Do these records include at least raw material source and storage, production batching, product quality test results and delivery details for all packaged and bulk feeds?
-
4.2.3 Are work instructions and manufacturing procedures regularly reviewed to ensure they remain effective?<br><br>-A regular review period for all procedures should be set. For example, every 3 years or upon changes to processes.
-
4.2.4 Are records of verification results for flushing and sequencing kept?<br><br>-Focus to be given to RAM and medication records.
4.3 SPECIFICATIONS
-
4.3.1 Do bag labels in use meet regulatory requirements, including reference to the restricted animal feeding ban?<br><br>-Refer to clause 2.10.<br>There needs to be a system of approving bag artwork prior to printing and after receiving new bags and tags to ensure all bags and tags meet regulatory requirements.
5 HAZARD RISK ASSESSMENT (HACCP)
5.1 HACCP TEAM
-
5.1.1 Is the HACCP team multi-disciplinary? <br>Are the team members trained in HACCP principles?<br><br>-FeedSafe training is adequate to meet this requirement. Refer to the SFMCA HACCP Instructional Videos.
-
5.1.2 Has a HACCP team leader been appointed? <br>Does this person have authority to perform the role?<br><br>-Refer to organisation chart and/or job descriptions for authorisations
5.2 HAZARD ASSESSMENT
-
5.2.1 Has a site hazard food safety risk assessment been completed and is it reviewed annually?<br><br>-A HACCP template is available on FeedSafe website resources section.
-
5.2.2 Does the risk assessment plan utilise HACCP principles, identifying risk areas and provide methods of managing these risks?<br><br>-Reference should be made to the Hazard Risk Assessment (HACCP) Support which identifies the major risks manufacturers need to manage. The seven principles of HACCP need to be used in managing risk hazards.
-
5.2.3 Has the process flow diagram been verified as accurate and includes all key steps?
-
5.2.4 Does the hazard assessment include:<br> Product descriptions,<br> Assessment of hazards at each step,<br> CCP identification?<br><br>-A HACCP template is available on FeedSafe website resources section.<br>All biological, chemical, and physical risks need to be assessed at each step.
5.3 CCP MANAGEMENT
-
5.3.1 Do CCP management records include:<br>• Measurable critical limit,<br>• Monitoring results,<br>• Responsibilities, and<br>• Planned corrective actions?<br><br>-Risks must be managed through identified critical control points that need to be integrated into the site’s operations. This needs to be confirmed during the audit process.
6 PURCHASING & SUPPLIERS
6.1 SUPPLIERS
-
6.1.1 Does the site maintain a register of compliant raw material suppliers?<br><br>-Refer to FeedSafe requirements for Supply Chain QA and FeedSafe Supply Chain QA Instructional Video.
-
6.1.2 Is there a documented purchasing program implemented with emphasis on raw material quality and safety risks?<br><br>-This needs to define how suppliers are approved and added to or removed from the approved supplier listing and who is authorised to approve new suppliers.
-
6.1.3 Is a copy of raw material purchasing standards kept on site; these may be GTA, other recognised industry standards or individual site acceptance standards?<br><br>-Refer to GTA Grain Commodity Vendor Declaration or equivalent where in use.
-
6.1.4 Does the purchasing standard or purchase contract include reference to grain treatment withholding periods?<br><br>-Refer to GTA Grain Commodity Vendor Declaration or equivalent where in use.
-
6.1.5 Are suppliers made aware of the quality standard in use and are they supplied with copies of the purchasing standard where appropriate?<br><br>-Refer to GTA Grain Commodity Vendor Declaration or equivalent where in use.
6.2 RECEIVALS
-
6.2.1 Is a record of the origin, date of receipt and quantities of each raw material received kept on file?
-
6.2.2 Is every load of incoming raw materials cross-referenced to purchasing documentation?
-
6.2.3 Where external third-party vehicles are delivering raw materials, is confirmation of what the previous load carried recorded?<br><br>-Use of transport driver declarations may be considered to confirm whether RAM has not been carried in the prior delivery. Attention is also to be given to contaminants such as glass, metal or chemical residues. What has been done if needed to decontaminate following the prior load. In some circumstances, confirmation of up to three prior loads may be required by some feed customers.
-
6.2.4 Are all received packaged raw materials adequately labelled (including ruminant feed warning statement) and in sound condition when received?<br><br>-Reference to the provision of RAM labelling requirement.
-
6.2.5 Are appropriate tests conducted when receiving raw materials (grains, soft meals, liquids, packaged materials)?<br><br>-Appropriate with respect to whether they meet purchase specification, this including visual inspection, sampling and testing. The testing needs to be linked to the food safety risk assessment (5.2) and defined critical control points (5.3).
-
6.2.6 Are raw materials found to be outside specification clearly identified and appropriately dealt with by authorised personnel?<br><br>-Confirm who is authorised to deal with this issue.
7 SAMPLING & TESTING
7.1 SAMPLING & TESTING PROGRAM
-
7.1.1 Does the site have a written raw material quality control program?
-
7.1.2 Does this program call for raw materials to be sampled and tested to ensure they comply with purchase contract and standard specifications?<br><br>-The HACCP Risk Assessment Plan should define the risks and raw materials requiring sampling and testing.
-
7.1.3 If samples are tested on site, are there testing procedures or protocols available and is equipment calibrated?<br><br>-Staff competency in sampling and testing as per clause 3.2.8.<br>Equipment maintenance and calibration as per clause 2.2.
-
7.1.4 Where samples are tested off site, is this conducted at a reputable external laboratory?<br><br>-The laboratory should reference a recognised methodology (eg NATA) on the analysis report. Additionally, the laboratory must have a certified practitioner of their science.
-
7.1.5 Are inspection results and tests assessed against documented tolerance/standards and records maintained?
7.2 RETENTION SAMPLES
-
7.2.1 Are retention samples of bulk raw materials taken and retained for at least three months?<br>Are retention samples identified or labelled to allow trace back to individual deliveries?<br><br>-Bulk materials risk assessed (clause 5.2) as not requiring sample retention should have justification provided based on supplier sampling and/or provision of lab or assay test results. <br>The three-month retention period is a minimum, for some higher risk raw materials retention for a minimum 6 months may be required to assist in any potential recalls.
-
7.2.2 Are retention samples of packaged raw materials taken and retained for at least three months?<br>Are retention samples identified or labelled to allow trace back to individual deliveries?<br><br>-Emphasis is to be given to bagged protein meals and raw materials that may vary with delivery and imported ingredients potentially subject to chemical residues.<br>It is acceptable to not store samples on site where the supplier has provided written assurance that they have retained samples of all products supplied e.g. some premix suppliers provide this sample retention service.<br>The three-month retention period is a minimum, for some higher risk raw materials more than 6 months may be required to assist in any potential recalls.
-
7.2.3 Are clearly labelled samples taken of all finished product bulk loads and packaged product runs, and retained for at least three months?<br>Is sampling of finished products conducted so that samples are sealed, separated, labelled and retained to allow easy retrieval?<br><br>-Preference is for a longer period, min. 6 months, in case of feed safety incidents and required traceability.
-
7.2.4 Are feed samples stored in appropriate conditions and can samples be easily retrieved?
7.3 VENDOR DECLARATIONS
-
7.3.1 Are stock food vendor declarations provided when requested by customers?<br><br>-Can be a separate form or a part of the delivery or invoicing documentation.
8 PRODUCTION
8.1 VALIDATIONS
-
8.1.1 Are there records confirming the mixer has been tested for mixing efficiency in the last 12 months?<br><br>-The intent is to have mixers that achieve a homogenous finished product. Regular mixer efficiency testing should be conducted, preferred 6 monthly checks.
-
8.1.2 Have cross-contamination measures been validated (e.g. flushing, sequencing) to ensure effective?<br><br>-Manufacturers must meet the maximum carry-over of certain coccidiostats as per (EU) No 574/2011.<br>Carryover testing records to be sighted, especially for RAM or medicated.
-
8.1.3 Is there a system to define how to set use by date periods for finished products?
8.2 MANUFACTURING
-
8.2.1 Are there written work instructions for the critical manufacturing process jobs?<br><br>-Work instructions need to include relevant responsibility for feed safety critical control points as per clause 5.3.
-
8.2.2 Is there a record of what is manufactured and is this also used to confirm any departure from the defined production procedure?
-
8.2.3 Are feed batching records kept which confirm that feed was manufactured according to formulation?
-
8.2.4 Are labelling and packaging materials assessed for quality before use?
-
8.2.5 Are there defined raw material weighing tolerances and are these monitored?<br><br>-For example, refer to equipment supplier specifications
-
8.2.6 Are bulk finished feeds correctly stored at the end of production to ensure separation and integrity of finished product?<br><br>-Storage bins, silos and tanks, labelling and identification as per clause 2.3.2<br>For silos and bin maintenance refer to clause 2.2.3. <br>
-
8.2.7 Are bagged finished products correctly packaged and labelled at the time of bagging?
-
8.2.8 Are there defined finished product weighing tolerances and are these monitored?<br><br>-Bag check weighing needs to ensure correct nett weights achieved. Refer NMI Guidelines on Check Weighing Products.
-
8.2.9 Is there a system of checking pallets prior to use to ensure they are in a clean and good physical condition and do not damage packaged products?
8.3 NON-CONFORMANCES
-
8.3.1 Are broken or damaged bags of finished product segregated and dealt with to ensure they are not supplied to clients?
-
8.3.2 Is there a method of investigation and corrective action when results are outside tolerance/standard?<br><br>-This should link to recall procedure, see clause 10.3.<br> This might be through the corrective action process as per clause 10.2.
8.4 REWORKS
-
8.4.1 Is there a procedure for labelling, storage and handling of reworks and returns?
-
8.4.2 Is there identification and disposal of classified waste products and are these labelled and segregated from raw materials and finished products?<br><br>-The intent is to prevent contamination of feed through the incorrect re-use of waste or other products. This does not stop the re-use of feed as long as it is done in a controlled manner. Audit focus should be placed on reviewing procedures in place to prevent RAM inclusion in ruminant feed and medication contamination via use of rework.
-
8.4.3 Is there approval for reworks release and reformulation by an authorised person?<br><br>-See clause 4.1 Formulations.
9 TRANSPORT
9.1 LOADING
-
9.1.1 Are there loading and delivery procedures for bulk and bagged products which ensures loading of delivery vehicles with the correct product, without risk of damage, unintended mixing or contamination?<br><br>-Delivery vehicle emphasis is on the trailer carrying feed. <br>Refer to Grain Industry, Transport Code of Practice
-
9.1.2 Is there a formal system of allocating finished product orders to out-loading bins and delivery vehicles?
-
9.1.3 Are all out-loading bins, transport vehicles and their compartments clearly identified through a labelling or numbering system?
9.2 TRANSPORT
-
9.2.1 Are delivery vehicles kept in clean, well maintained and roadworthy condition, and designed such that feeds can be kept dry and protected from contamination during transport and delivery?
-
9.2..2 Are bulk and bagged product transport vehicle loads covered during delivery?
-
9.2.3 If delivery vehicles are involved in any incident (e.g. accident) which could result in feed contamination, is there a system for reporting and determining the resulting actions regarding subsequent product delivery, return or disposal?
9.3 BULK DELIVERY
-
9.3.1 Does bulk delivery and/or invoice documentation meet regulatory requirements, with specific reference to the restricted animal feeding ban?<br><br>-Refer to clause 2.10.
-
9.3.2 Are delivery vehicles inspected prior to loading to ensure they do not contain feed residues which can contaminate subsequent deliveries?<br>If residues are found are cleaning procedures in place?<br><br>-Emphasis on RAM and medicated feeds and out-loading bins and vehicles where the next load is a non-medicated feed.
-
9.3.3 Are bulk vehicles which have carried feed containing restricted animal materials cleaned prior to loading ruminant feeds?
-
9.3.4 Where external third-party vehicles are loaded, is confirmation of what the previous load carried obtained?<br><br>-Use of transport driver declarations should be considered to confirm whether RAM has not been carried in the prior delivery. In some circumstances, confirmation of up to three prior loads may be required by some customers.
-
9.3.5 Are documents provided to transport drivers to identity the feed products in a given load (by compartment as applicable) and clear instructions as to the precise destination for delivery of each product?
-
9.3.6 Are feed clients reminded of their responsibility to provide adequate, safe and unobstructed facilities for unloading, and the clear and visible identification of all their storage facilities (silos, bins, etc.)<br><br>-This can be in the form of a memo, newsletter and/or part of the customer delivery paperwork. Refer to Silo Safety Alert advice.
-
9.3.7 Are bulk feed products delivered into correctly identified farm storage facilities?<br><br>-Drivers should be trained in delivery procedures and actions to take if bulk silos are unacceptable, delivery instructions are inadequate, or feed will not fit into the designated silo.
-
9.3.8 Product is not unloaded into alternative facilities unless specifically permitted by the recipient and documented?<br><br>-Need to have been included within delivery driver training.
-
9.3.9 Do drivers inspect truck compartments to ensure complete emptying and report/record instances of incomplete unloading?<br><br>-Returned feed should be cross referenced to return weighbridge documentation.
-
9.3.10 Is any significant spillage reported to the mill site and the customer, and the spilt feed disposed of?<br><br>-Procedures need to be linked to customer complaint management system.
10 MONITORING & IMPROVEMENT
10.1 CUSTOMER COMPLAINTS
-
10.1.1 Is there a written customer complaint procedure for registering and investigating problems?<br><br>-This should link to recall procedure, see clause 10.3.
-
10.1.2 Is there a record of timely resolution of complaints and identification of non-conformances which lead to corrective actions?<br><br>-Customer complaint procedures should be resulting in continuous improvement in manufacturing processes, products and services.
10.2 CORRECTIVE ACTIONS
-
10.2.1 Is there a written process for recording and monitoring corrective actions?<br><br>-It is expected there is a process for investigating root causes of any recalls, complaints, non-conformances, internal audit findings, etc.
10.3 RECALLS
-
10.3.1 Is there a written product recall procedure which is linked to the customer complaint procedure?<br><br>-See clause 10.1.
-
10.3.2 Does the recall system apply in other circumstances (e.g. product found to be out of specification), not just customer complaints?<br><br>-A proactive system to respond to non-conforming products rather than relying on customer complaints.
-
10.3.3 Is there a site Recall Committee with clearly defined members and documented responsibilities?<br><br>-Emphasis is placed on having a process of handling non-conforming product and staff responsible for acting when non-conforming product is identified.
-
10.3.4 Does the recall procedure include emergency and out of hours contact persons and telephone numbers?<br><br>-Emphasis is placed on having a process of handling non-conforming product and staff responsible for acting when non-conforming product is identified.
-
10.3.5 Does the recall procedure call for: prompt retrieval of hazardous products from the marketplace, notification of relevant government authorities and minimisation of disruption to end-users of products?<br><br>-Emphasis is placed on having a process of handling non-conforming product and staff responsible for acting when non-conforming product is identified.
-
10.3.6 Does the recall procedure specify methods to identify, locate and control recalled product and to isolate recalled product on return to the mill?
-
10.3.7 Is each recall incident documented and reviewed to ensure procedures were adequate?
-
10.3.8 Are mill practices and procedures reviewed to prevent recurrence?<br><br>-This should link to corrective actions clause 10.2.
-
10.3.9 Is the recall system periodically reviewed/tested for its effectiveness?<br><br>-Periodically is taken as being a minimum annual review.
10.4 INTERNAL AUDITS
-
10.4.1 Are internal audits undertaken to ensure the requirements within this Audit Checklist are being met between annual FeedSafe audits.<br><br>-This Audit Checklist needs to be used to conduct internal audits through the year to ensure the compliance standard is being met. There needs to be a record that internal audits have been undertaken.<br>The annual audit must confirm that internal audits have been undertaken to cover the whole quality and feed safety system at least once per year, with this being more than 3 months before or after the annual FeedSafe audit.
11 BIOSECURITY
-
11.1.1 Is there a system to co-ordinate delivery vehicle movements in the event of a notifiable or emergency disease outbreak in the area within which feed is delivered?<br><br>-Refer to the National Biosecurity Manual for Feed Mills for information on Emergency Disease Action Plans.
-
11.1.2 Are customer quarantine/biosecurity measures known and adhered to by the mill and drivers?
PART 3 NON-COMPLIANCE REVIEW
- Non-Compliance Review
-
NC No
-
Standard Reference
-
Detail of non-conformity
-
Grade of NC (e.g. Minor)
-
Action Due Date
-
Corrective Action taken & evidence provided
-
Satisfactory
-
Close out Date
PART 4 REPORTING
Assessment of Hazard Risk - Code of GMP Items Presenting a Non-compliance
-
Major non-compliance: The auditor believes that the point of non-compliance results in a high risk that finished products present a hazard to animal health and human food products. It is expected that all questions shown with a “must” priority will be present within the sites QA program.
For example: 2.10.1 If there is not a separate receiving hopper for RAM, are written procedures in place to prevent cross contamination of received raw materials? If the company has no written procedures to prevent RAM cross transference, this is classified as a major non-compliance.
Moderate non-compliance: The auditor believes that the point of non-compliance results in a moderate risk that finished products present a hazard to animal health and human food products. For “must” questions where companies cannot demonstrate that they are following their program, then this is expected to be classified as a moderate non-compliance.
For example: 2.10.1 If there is not a separate receiving hopper for RAM, are written procedures in place to prevent cross contamination of received raw materials? If the company has written procedures to prevent RAM cross transference but cannot provide evidence that they are following these procedures this is classified as a moderate non-compliance.
Minor non-compliance: The auditor believes that the point of non-compliance presents a low risk that finished products present a hazard to animal health and human food products. For example: Where a CCP document is found as not having been completed in one instance but usually is, this is seen as a minor NCR.
Repeat non-compliance: Where there has been a repeated non-compliance or an observation not addressed at a subsequent audit, these are automatically upgraded to the next non-compliance level. For example: a moderate would become a major. -
Auditors are encouraged to use the comments box to share any recommendations for continuous improvement, however this must not replace a non-compliance.
-
Once the audit has been completed, the auditor is required to complete the following FeedSafe Audit Statement. Audit Reports and Statements are to be sent to contact@sfmca.com.au within 5 calendar days of audit as well as the updated versions within 5 calendar days of non-compliance closeout evidence provided by manufacturer.
Note this statement must be completed and signed by the auditor. SFMCA does not accept alternate statements from auditors.
A separate Audit Report and Statement is required for each manufacturing site.
FeedSafe Audit Timeline
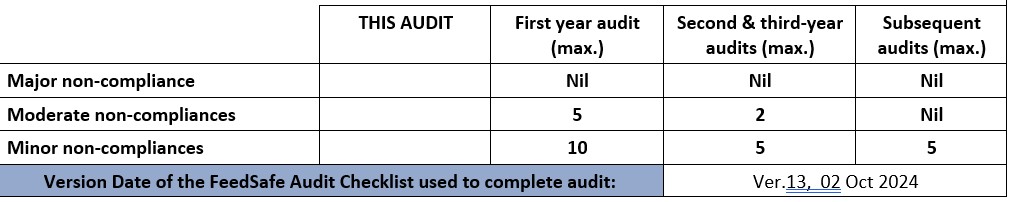
FeedSafe Audit Statement Ver.13
-
I ___________________________, as an accredited Exemplar Global Food Safety auditor have completed an audit on the stockfeed manufacturing site listed below against the FeedSafe Audit Checklist and confirm that this site achieved the following outcome:
-
-
Major non-compliance : This audit
-
Moderate non-compliances : This audit
-
Minor non-compliances : This audit
-
NOTE: FeedSafe certification can continue while manufacturer closes out non-compliances if the above criteria is met. If this audit non-compliances are more than the relevant section of the table above, then certification cannot be granted until the non-compliances are close out to the satisfaction of the auditor and FeedSafe Man.
-
Company Name:
-
(Company being audited, ensure this is accurate as this will appear on FeedSafe certificates)
-
Company Postal Address:
-
Site Physical Address:
-
Audit Date:
-
I declare that I am a FeedSafe approved, third-party auditor, not being an employee of the company or having worked in a consulting capacity to assist the company in implementing their QA program.
-
Signed:
-
Date:
-
Exemplar Global Auditor No:
-
Auditor’s Name:
-
Address:
-
Email Address:
-
Phone No.
SIGN OFF
-
Auditor