Title Page
-
Final Audit Score
- 0
- .25
- .50
- .75
- 1.00
- 1.25
- 1.50
- 1.75
- 2.00
- 2.25
- 2.50
- 2.75
- 3.00
- 3.25
- 3.50
- 3.75
- 4.00
- 4.25
- 4.50
- 4.75
- 5.00
- 5.25
- 5.50
- 5.75
- 6.00
- 6.25
- 6.50
- 6.75
- 7.00
- 7.25
- 7.50
- 7.75
- 8.00
- 8.25
- 8.50
- 8.75
- 9.00
- 9.25
- 9.50
- 9.75
- 10.00
- 10.25
- 10.50
- 10.75
- 11.00
- 11.25
- 11.50
- 11.75
- 12.00
- 12.25
- 12.50
- 12.75
- 13.00
- 13.25
- 13.50
- 13.75
- 14.00
- 14.25
- 14.50
- 14.75
- 15.00
- 15.25
- 15.50
- 15.75
- 16.00
- 16.25
- 16.50
- 16.75
- 17.00
- 17.25
- 17.50
- 17.75
- 18.00
- 18.25
- 18.50
- 18.75
- 19.00
- 19.25
- 19.50
- 19.75
- 20.00
- 20.25
- 20.50
- 20.75
- 21.00
- 21.25
- 21.50
- 21.75
- 22.00
- 22.25
- 22.50
- 22.75
- 23.00
- 23.25
- 23.50
- 23.75
- 24.00
- 24.25
- 24.50
- 24.75
- 25.00
- 25.25
- 25.50
- 25.75
- 26.00
- 26.25
- 26.50
- 26.75
- 27.00
- 27.25
- 27.50
- 27.75
- 28.00
- 28.25
- 28.50
- 28.75
- 29.00
- 29.25
- 29.50
- 29.75
- 30.00
- 30.25
- 30.50
- 30.75
- 31.00
- 31.25
- 31.50
- 31.75
- 32.00
- 32.25
- 32.50
- 32.75
- 33.00
- 33.25
- 33.50
- 33.75
- 34.00
- 34.25
- 34.50
- 34.75
- 35.00
- 35.25
- 35.50
- 35.75
- 36.00
- 36.25
- 36.50
- 36.75
- 37.00
- 37.25
- 37.50
- 37.75
- 38.00
- 38.25
- 38.50
- 38.75
- 39.00
- 39.25
- 39.50
- 39.75
- 40.00
- 40.25
- 40.50
- 40.75
- 41.00
- 41.25
- 41.50
- 41.75
- 42.00
- 42.25
- 42.50
- 42.75
- 43.00
- 43.25
- 43.50
- 43.75
- 44.00
- 44.25
- 44.50
- 44.75
- 45.00
- 45.25
- 45.50
- 45.75
- 46.00
- 46.25
- 46.50
- 46.75
- 47.00
- 47.25
- 47.50
- 47.75
- 48.00
- 48.25
- 48.50
- 48.75
- 49.00
- 49.25
- 49.50
- 49.75
- 50.00
- 50.25
- 50.50
- 50.75
- 51.00
- 51.25
- 51.50
- 51.75
- 52.00
- 52.25
- 52.50
- 52.75
- 53.00
- 53.25
- 53.50
- 53.75
- 54.00
- 54.25
- 54.50
- 54.75
- 55.00
- 55.25
- 55.50
- 55.75
- 56.00
- 56.25
- 56.50
- 56.75
- 57.00
- 57.25
- 57.50
- 57.75
- 58.00
- 58.25
- 58.50
- 58.75
- 59.00
- 59.25
- 59.50
- 59.75
- 60.00
- 60.25
- 60.50
- 60.75
- 61.00
- 61.25
- 61.50
- 61.75
- 62.00
- 62.25
- 62.50
- 62.75
- 63.00
- 63.25
- 63.50
- 63.75
- 64.00
- 64.25
- 64.50
- 64.75
- 65.00
- 65.25
- 65.50
- 65.75
- 66.00
- 66.25
- 66.50
- 66.75
- 67.00
- 67.25
- 67.50
- 67.75
- 68.00
- 68.25
- 68.50
- 68.75
- 69.00
- 69.25
- 69.50
- 69.75
- 70.00
- 70.25
- 70.50
- 70.75
- 71.00
- 71.25
- 71.50
- 71.75
- 72.00
- 72.25
- 72.50
- 72.75
- 73.00
- 73.25
- 73.50
- 73.75
- 74.00
- 74.25
- 74.50
- 74.75
- 75.00
- 75.25
- 75.50
- 75.75
- 76.00
- 76.25
- 76.50
- 76.75
- 77.00
- 77.25
- 77.50
- 77.75
- 78.00
- 78.25
- 78.50
- 78.75
- 79.00
- 79.25
- 79.50
- 79.75
- 80.00
- 80.25
- 80.50
- 80.75
- 81.00
- 81.25
- 81.50
- 81.75
- 82.00
- 82.25
- 82.50
- 82.75
- 83.00
- 83.25
- 83.50
- 83.75
- 84.00
- 84.25
- 84.50
- 84.75
- 85.00
- 85.25
- 85.50
- 85.75
- 86.00
- 86.25
- 86.50
- 86.75
- 87.00
- 87.25
- 87.50
- 87.75
- 88.00
- 88.25
- 88.50
- 88.75
- 89.00
- 89.25
- 89.50
- 89.75
- 90.00
- 90.25
- 90.50
- 90.75
- 91.00
- 91.25
- 91.50
- 91.75
- 92.00
- 92.25
- 92.50
- 92.75
- 93.00
- 93.25
- 93.50
- 93.75
- 94.00
- 94.25
- 94.50
- 94.75
- 95.00
- 95.25
- 95.50
- 95.75
- 96.00
- 96.25
- 96.50
- 96.75
- 97.00
- 97.25
- 97.50
- 97.75
- 98.00
- 98.25
- 98.50
- 98.75
- 99.00
- 99.25
- 99.50
- 99.75
- 100.00
- 100.25
- 100.50
- 100.75
- 101.00
- 101.25
- 101.50
- 101.75
- 102.00
- 102.25
- 102.50
- 102.75
- 103.00
- 103.25
- 103.50
- 103.75
- 104.00
- 104.25
- 104.50
- 104.75
- 105.00
- 105.25
- 105.50
- 105.75
- 106.00
- 106.25
- 106.50
- 106.75
- 107.00
- 107.25
- 107.50
- 107.75
- 108.00
- 108.25
- 108.50
- 108.75
- 109.00
- 109.25
- 109.50
- 109.75
- 110.00
- 110.25
- 110.50
- 110.75
- 111.00
- 111.25
- 111.50
- 111.75
- 112.00
- 112.25
- 112.50
- 112.75
- 113.00
- 113.25
- 113.50
- 113.75
- 114.00
- 114.25
- 114.50
- 114.75
- 115.00
- 115.25
- 115.50
- 115.75
- 116.00
- 116.25
- 116.50
- 116.75
- 117.00
- 117.25
- 117.50
- 117.75
- 118.00
- 118.25
- 118.50
- 118.75
- 119.00
- 119.25
- 119.50
- 119.75
- 120.00
- 120.25
- 120.50
- 120.75
- 121.00
- 121.25
- 121.50
- 121.75
- 122.00
- 122.25
- 122.50
- 122.75
- 123.00
- 123.25
- 123.50
- 123.75
- 124.00
- 124.25
- 124.50
- 124.75
- 125.00
- 125.25
- 125.50
- 125.75
- 126.00
- 126.25
- 126.50
- 126.75
- 127.00
- 127.25
- 127.50
- 127.75
- 128.00
- 128.25
- 128.50
- 128.75
- 129.00
- 129.25
- 129.50
- 129.75
- 130.00
- 130.25
- 130.50
- 130.75
- 131.00
- 131.25
- 131.50
- 131.75
- 132.00
- 132.25
- 132.50
- 132.75
- 133.00
- 133.25
- 133.50
- 133.75
- 134.00
- 134.25
- 134.50
- 134.75
- 135.00
- 135.25
- 135.50
- 135.75
- 136.00
- 136.25
- 136.50
- 136.75
- 137.00
- 137.25
- 137.50
- 137.75
- 138.00
- 138.25
- 138.50
- 138.75
- 139.00
- 139.25
- 139.50
- 139.75
- 140.00
- 140.25
- 140.50
- 140.75
- 141.00
- 141.25
- 141.50
- 141.75
- 142.00
- 142.25
- 142.50
- 142.75
- 143.00
- 143.25
- 143.50
- 143.75
- 144.00
- 144.25
- 144.50
- 144.75
- 145.00
- 145.25
- 145.50
- 145.75
- 146.00
- 146.25
- 146.50
- 146.75
- 147.00
- 147.25
- 147.50
- 147.75
- 148.00
- 148.25
- 148.50
- 148.75
- 149.00
- 149.25
- 149.50
- 149.75
- 150.00
- 150.25
- Exceeded max score of 150.00
-
Site
-
Facility
-
Conducted on
-
Participants
-
Assessor(s)
-
Assessor(s)
-
Type of Audit
- On-site
- Desktop
- Remote
Final Audit Score Outline
Final Audit Score Outline
-
Total Number of Recommendations
- 0
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
-
Total Number of Minors
- 0
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
-
Total Number of Majors
- 0
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
-
Total Number of Criticals
- 0
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
-
Total Number of Repeat Findings
- 0
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
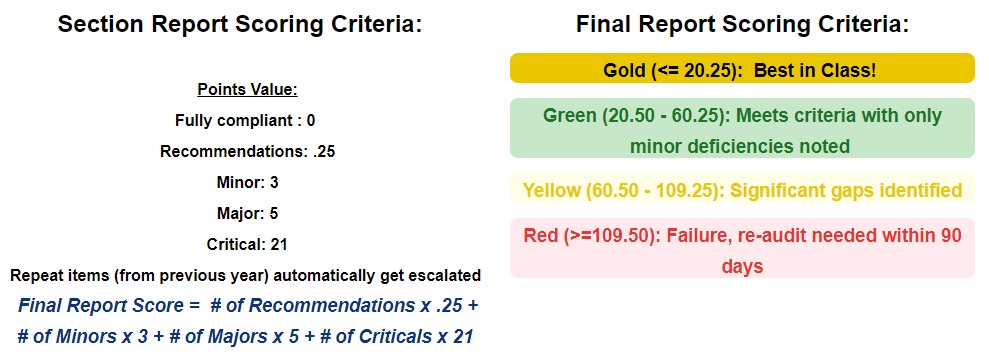
Scoring Summary
Scoring Information
-
-
If not corrected, repeated findings from the previous year will result in an escalation.
Example: Section 7.2 .2 documented in 2022 as a Recommendation. Section 7.2.2 observed in 2023 as a Recommendation but due to this being a repeat it will be documented as a Minor. -
A Critical finding results in an auto-failure for both the section and overall assessment. A re-assessment is required within 90 days.
1.0 Senior Management Commitment
1.1 Management Responsibility
-
1.1.1 The site shall have a documented Food Safety, Quality and Regulatory Compliance Policy Statement which states the site's commitment to meet its obligation to produce and supply safe, legal, and authentic products to the specified quality and its responsibility to its customers. The policy includes: - site's intention to comply with all customer and regulatory requirements in the country where products are manufactured and also in markets products are exported to - site's commitment to continually improve food safety, quality and regulatory compliance management systems -site's commitment to assess and improve the food safety and quality culture -site's commitment to effective food defense and food fraud prevention. This Policy Statement shall: -be signed by the person with overall responsibility for the site -be displayed in prominent locations and effectively communicated to all site personnel in the languages understood by all site personnel -be reviewed at least once per year or when major changes are made in the organization. Method used to communicate the policy shall be stated in the Policy Statement. All employees shall be aware of the policy and should be able to demonstrate understanding of the Policy Statement. Written communication from managment to production employees encouraging feedback is present. Note: Even if the company has a company-wide FSQR Policy, the local facility must have a facility Policy Statement. Identification of objectives, key indicators and method to measure them. Senior management shall designate PCQI practitioner.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive Action -
-
Repeat
-
Corrective action -
-
Preventive action -
-
Repeat
-
Corrective Action -
-
Preventive Action -
-
Repeat -
-
1.1.2 The company's senior management shall provide, document human & financial resources required to implement and improve the processes of the quality management, food safety / HACCP plan, infrastructure and quality culture. This includes: - availability of resources - adequate staffing - skill and competency level - training & documentation and resources including: - maintenance for production - suitable equipment - allocations of budget for food safety - HACCP training. The food safety and quality systems are designed, implemented and maintained. The plan shall also include behaviors needed to achieve the intended positive culture change. This shall include: - defined activities involving all sections of the site that have an impact on product safety. As a minimum, these activities shall be designed around: - clear and open communication on product safety - feedback from employees - behavior changes required to improve product safety processes - performance measurement on product safety, authenticity, legality and quality related activities - an action plan indicating how the activities will be undertaken and measured, and the intended timescales - a review of the effectiveness of completed activities. Site management shall ensure departments and operations are appropriately staffed.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
1.1.3 There shall be clear communication & reporting channels to Sr. management for departments responsible for monitoring compliance. Each department responsible for compliance shall show evidence that they regularly report compliance to and deviations from targets. These objectives shall be: - documented and include targets or clear measures of success - clearly communicated to relevant staff - monitored and results reported at least quarterly to site senior management and all staff.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
1.1.4 Senior Management shall establish food safety, authenticity, legality and quality objectives. These objectives are established, documented, monitored and reviewed at least quarterly with relevant staff. Significant food safety metrics shall inlcude: - environmental analysis - operators hygiene measures - microbiological analysis/ pathogen testing on products - identification of food safety risk /zones - customer complaints - continuous improvement
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
1.1.5 Senior Management shall ensure that there is a process to identify and address any safety, legal or regulatory issues at a strategic level. There shall be clear communication & reporting channels to Sr. management for departments responsible for monitoring compliance. Each department responsible for compliance shall show evidence that they regularly report compliance to and deviations from targets. Targets for food safety & quality and other key performance indicators, such as cost and productivity are equally measured. The site shall have a demonstrable meeting program which enables food safety, authenticity, legality, and quality issues to be brought to the attention of senior management. These meetings shall occur at least monthly. The company’s senior management shall have a process for assessing any concerns raised. Records of the assessment and, where appropriate, actions taken, shall be documented. Serious issues shall be addressed at the Senior Management level up to Corporate office. Implications to the company’s business are adequately addressed so changes may be made when necessary.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
1.1.6 Organization chart & supporting documents shall provide a snapshot of how positions interact & share responsibility for Food Safety. (Org. chart, Job descriptions & backup personnel or other on a separate document). The interrelationship between FS & Quality must be defined. It shall be clearly documented who deputizes in the absence of the responsible person. A process is in place to ensure the integrity of food safety & quality management systems is maintained in the event of change within the company. Includes organizational or management change. Job descriptions that define food safety and quality responsibilities shall be documented (personnel performing key process steps and responsible for achieving quality requirements shall be documented and include provisions for coverage in the absence of key personnel). - The supplier will establish a food safety culture program at all levels of the organization. Senior management will provide evidence of the commitment to implementing and maintaining the organization's food safety culture. - Guidelines: Personnel roles, responsibilities and authorities for food safety and quality management are well defined, communicated and understood. - A member of the site's management team (and a designee) must be nominated having overall responsibility for compliance with SQMS standards and McDonald's Global and Market requirements.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
1.1.7 There shall be a current version of any standards the site is audited against (BRCGS, Conagra, YUM!, GSF Readiness Assessment, SQF, Global GAP, or other programs approved by the customer.etc.).
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
1.1.8 Most senior Production or Operations manager shall participate in the opening and closing meetings of this assessment and be available during the audit for a discussion on effective implementation of the food safety and quality culture plan.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
1.1.9 Operations manager or above shall accompany auditor during facilities walkthrough. The site is expected to be prepared to meet requirements at all times and to have appropriate team members available.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
1.1.10 Operations, QA or any manager shall accompany auditor during QA & Sanitation walkthroughs.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
1.1.11 Last GFSI audit CAPA completed according to established timeline. Note: Verification of CAPA to be performed during the plant visit.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
1.1.12 Previous year's Readiness Assessment CAPA was submitted within 28 calendar days after final report was issued (variances requested and proof present, if applicable). All corrective actions have been completed according to established timelines.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
1.2 Management Review
-
1.2.1 Senior management shall take responsibility for the review process, ensuring that a complete management review of the Food Safety (including HACCP) & Quality Systems occurs. All management reviews must have a formal agenda to cover the following items as a minimum: - customer requirements - operations - maintenance - effectiveness and - ongoing improvement The review process shall be undertaken at planned intervals, at a minimum of annually, to insure critical evaluation of the food safety plan & the HACCP system's suitability, adequacy and effectiveness.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
1.2.2 Records of management reviews shall be comprehensively documented and retained. Reviews shall include, at a minimum: - internal, second party and third party audits - previous management review documents - action plans & timeframes - customer performance indicators - complaints and feedback - incidents (including both recalls and withdrawals) - corrective actions - out of specification results & non-conforming materials - process performance and deviation from defined parameters - reviews of the HACCP based system - review of latest quality culture surveys - food defense and authenticity - quality culture - previous managment review and action plan - legislative compliance - to include changes (and upcoming/potential changes) to legislation. - developments in scientific information associated with the products within the scope - customer specifications - microbiological results including the enviornmental monitoring program - allergen verification results (where applicable - foreign material results - emerging risks - resource requirements. Corrective actions, and timescales for their implementation, shall be agreed and their completion verified. As a minimum a summary of the results shall be reviewed in the management review meetings
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat
-
Corrective action -
-
Preventive action -
-
Repeat -
-
1.2.3 Decisions and actions agreed upon within the review process shall be effectively communicated to appropriate staff, and actions implemented within agreed timeframes. Records shall be updated to show when actions have been completed. As a minimum the following must be included: - formal minutes - action tracker and timelines - specific responsibilities (single person) for all actions
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
2. 0 HACCP/Food Safety Program
2.1 HACCP/Food Safety Team
-
2.1.1 - A HACCP Food Safety Team is formed consisting of cross functional relevant skills and knowledge. - The HACCP Food Safety Team members have documented qualifications and training in the principles of HACCP. - A HACCP Food Safety Team Leader is present and has documentation of competency. This is by documentation of an industry recognized HACCP training course and demonstrated knowledgeable of the HACCP Plan and systems. - The lead individual who is responsible for the maintenance and updating of the HACCP program has had formal HACCP (or PCQI) training - For FDA plants, the Team leader is a Certified PCQI (Preventative Control Qualified Individual). - For sites complying with SQF criteria, the facility is to have a list of all McD's product made at the facility being audited. A Primary and substitute SQF practioner shall be designated for each site.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
2.1.2 The HAACP Food Safety Team or designated person meets at a frequency that is sufficient to maintain the Plan(s), and when any changes in: -operations -structure -engineering or -formulation occur which may affect Food Safety. The process includes changes to raw ingredients and packaging that may affect Food Safety. The team's activities and meetings shall be documented and communicated to relevant personnel.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat
-
Corrective action -
-
Preventive action
-
Repeat -
-
2.1.3 The HACCP Food Safety Team shall verify and validate the HACCP/Food Safety Plan a minimum of once per year even if there are no changes. This shall be documented and include: - Flow Diagram - CCPs - Critical Limits - Corrective Actions - Monitoring procedures - Scientific literature and - Validation. This includes any change to process flow, emergence of a known adulteration of an ingredient, following a recall or as identified as an emerging risk. A written revision history that includes the reason for the change(s) shall be maintained. Verification shall occur annually or when any of the following occur, but is not limited to: - Change in ingredients and/or recipe, - Change in processing conditions - Process flow or equipment, - Updated or new processing line for product - Change in food contact packaging - Storage or distribution conditions - Change in consumer use of finished product - Emergence of a new risk (e.g. known adulteration of an ingredient) - Responding to customer complaint reviews to prevent reoccurrence - Following a recall/withdrawal - New developments within industry including scientific information associated with ingredients, process or product. Food Safety Risk Assessments must addresses all potential hazards. Includes hazards identified by HACCP program and also potential hazards related to and include, but are not limited to: - Food allergens - Foreign material - Sanitation - Suppliers - Environment and others. A risk assessment is a thorough evaluation of the likelihood that a foreseeable hazard could be present and the severity this would have on a consumer. The HACCP food safety team shall specify and document the corrective action to be taken when monitored results indicate a failure to meet a control limit, or when monitored results indicate a trend towards loss of control. See RA 2.2.7 Dairy: YUM! FSAL 13.2 5 Only dairy products produced from pasteurized milk shall be used. In any case where unpasteurized dairy is considered, a risk assessment is required and use shall be approved by the Global Food Safety Council
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
2.1.4 The plan must be verified and validated before the product is mass produced. The food safety team shall validate all of the critical limits to ensure the level of control of the identified food safety hazard(s) and that all critical limits and control measures individually or in combination effectively provide the level of control required Validation includes (this is not an exhaustive list): - Internal audits - Review of records where acceptable limits have been exceeded - Review of compaints by enforcement authorities or customers - Review of incidents of product withdrawel or recall. Results shall be recorded and communicated.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
2.1.5 All personnel who enter production areas should have adequate training about the importance of health and hygiene in preventing contamination of food and food production areas; - Human resource personnel should have training adequate to enable them to ensure that all personnel receive training necessary to perform their assigned functions. - Personnel with responsibilities for purchasing should have adequate training regarding the importance of applicable food safety specifications and supply-chain controls (with associated supplier approval and verification activities) for the raw materials and other ingredients that they purchase. - Personnel with responsibilities for receiving raw materials and other ingredients should have adequate training regarding the importance of inspecting shipments at receipt and using proper storage conditions, including when applicable refrigeration and segregation (e.g.,when applicable, segregation of raw foods from RTE foods and segregation of foods with different food allergen profiles). - Personnel with responsibilities for maintenance should have adequate training regarding the potential for maintenance activities to introduce contamination or lead to allergen cross-contact. - Personnel with responsibilities for cleaning and sanitizing the plant, including food-contact surfaces, should have adequate training regarding each step in the cleaning/sanitizing process and actions to take if there are visual observations or other indication that a step was not performed correctly or was not effective. - Personnel with responsibilities for food production should have adequate training in the procedures for food production, including the importance of each step in production and actions to take if there is a problem during production. - Personnel with responsibilities for sampling surfaces for environmental pathogen should have adequate training in the procedures for collecting the samples. - Personnel with responsibilities for laboratory testing of food or environmental samples should have adequate training in conducting that laboratory testing. - Supervisory personnel should have adequate training in the procedures, included in the food safety plan, applicable to their supervisory responsibilities. - Plant management should have adequate training in the food safety plan as a whole.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
2.1.6 All management demonstrates knowledge of the HACCP/Food Safety plans and CCPs in each of their areas of responsibilities. The relationship between HACCP and legal compliance is understood. Employees monitoring CCPs are aware of CCPs and the critical limits in their area(s) and what action to take if the limit(s) are exceeded. (Determined through both interview and training records review). The HACCP / Food Safety plan team will be assessed.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
2.2 HACCP/Food Safety Plan follows Codex or NACMF requirements
-
2.2.1 A description of the product is present considering all aspects that may affect food safety. The description/scope contains: - Composition (ingredients) - Target consumer - Consideration of vulnerable populations (infants, elderly, allergen suffers) - Physical and chemical properties impacting food safety - Processing - Packaging - Storage - Distribution - Shelf life - Instructions for use (intended use as well as any alternative uses), and - Consider misuse. - Country of Origin for Raw Materials and Packaging (if filed in external source, then must specify) Where any processes are outsourced, including production, manufacture, processing or storage, the risks to the product safety, authenticity, integrity, legality and quality shall form part of the site’s food safety plan (HACCP plan). For SQF Facilities ONLY: Shall include a list of the products covered under the scope of the certification.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
2.2.2 An accurate flow diagram of the process containing all inputs and outputs, and CCP's is present. The HACCP food safety team shall verify the accuracy of the flow diagrams by on-site audit and challenge at least annually and whenever there are changes which may affect food safety. CCP (s) are identified in the process flow chart. Documentation of verification by the HACCP Food Safety Team is present for the diagram. Current flow diagram is signed & dated by HACCP team members. Included should be: -Site map of the premises, including equipment layout -Water distribution diagram for the site, including steam. - Raw Materials - Utilities - Packaging -Area zones (including high risk, high-care or ambient high-care) -Intermediate / semi-processed products -By-products (e.g. rework, recycle) -Waste. GSF Corp: Pre-weigh / Kitting process to be included in the process flow. Some of the components don't have to be in the actual HACCP/Food Safety plan on file, it could be part of the verification and validation by the team.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
2.2.3 Documented, risk based hazard analysis for each process step identifying all potential hazards and measures to control the hazards is present. Justification for exclusion or evidence of the effectiveness of an alternative control measure must be documented. Hazards to be considered include: -Microbiological: Microbiological contamination control measures must be validated, documented, and implemented for any known and potential microbiological hazards. The control measures shall be reviewed whenever there are changes to the product, processes, or when a new hazard is identified in the industry. -Physical contamination -Chemical -Radiological contamination - for incoming ingredients and facility location to radiological sites -Fraud (e.g. substitution or deliberate/intentional adulteration) -Malicious contamination of products -Allergen risks It shall also take account of the preceding and following steps in the process chain. Hazard analysis shall take into account: -Likely occurrence of hazard -Severity of the effects on consumer safety -Vulnerability of those exposed -Survival and multiplication of micro-organisms of specific concern to the product -Presence or production of toxins, chemicals or foreign bodies -Contamination of raw materials, intermediate/semi-processed product, or finished product.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
2.2.4 Risk assessments shall contain: -Ingredients (or group of raw materials), -Packaging (Food Contact) -Allergens (content or potential contamination) -Country of origin. Consider: foreign-body risks -Microbiological contamination -Chemical contamination -Variety or species cross-contamination, -Substitution or fraud -The storage location of where these assessments are filed needs to be documented in the HACCP / Food Safety Plan. -Risk Assessment justifications and hazards must match the Hazard Analysis if it is deemed a hazard. -Any risks associated with raw materials which are subject to legislative control or customer requirements. -Reference to reviewing FDA Appendix 1 needs to be documented in the HACCP / Food Safety Plan. Consideration shall also be given to the significance of a raw material to the quality of the final product. The risk assessment shall form the basis for the raw material acceptance and testing procedure and for the processes adopted for supplier approval and monitoring. The risk assessment for a raw material shall be updated when: -There is a change in a raw material or the processing of a raw material or the supplier of a raw material, if a new risk emerges, following a product recall or withdrawal, where a specific raw material has been implicated, at least every 3 years. Note: Country of origin is important for consideration of fraud. The site must have a written Vulnerability Assessment Critical Control Points (VACCP) process that identifies vulnerabilities (such as the potential for counterfeiting, dilution, mislabeling and intentional Adulteration) along with associated mitigations or corrective actions. The methodology used to develop the VACCP plan must be traceable to recognized science. The assessment shall be documented and reviewed periodically.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
2.2.5 CCP's/Preventative Controls shall be established at the proper process step with a critical limit to control the hazard. A documented monitoring procedure for all CCPs and preventive controls (if facility is under US FDA's jurisdiction) shall be present. For each CCP, the appropriate critical limits shall be defined in order to identify clearly whether the process is in or out of control. Critical limits shall be: - measurable wherever possible (e.g. time, temperature, pH) - supported by clear guidance or examples where measures are subjective (e.g. photographs)
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
2.2.6 Validation is present for all CCP's/Preventative Controls and Critical Limits. Literature, challenge studies, in-process studies, etc. can all be used to validate CCPs/Preventative Controls. Validation must be present whenever the HACCP Food Safety Plan is changed. See RA 2.1.4 for details. The HACCP plan is validated, revalidated, and reviewed when product/process changes occur. If there have been no changes, the HACCP plan must be reviewed annually. Records are maintained.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
2.2.7 Corrective Actions for any deviations beyond the critical limits are documented. Methods & responsibilities outlining how immediate corrections & corrective actions are investigated / resolved / managed shall be in evidence. Corrective Actions include root cause analysis and a permanent corrective actions. Records are analyzed for trends, then reviewed and signed by qualified management staff, to prevent reoccurrence.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
2.2.8 Verification procedures are written for the HACCP Food Safety. Plan to include: - Flow Diagrams - Validation - Scientific data - CCP's / Preventative Controls - Monitoring effectiveness completed a minimum of yearly. Other verifications such as line audits and records may occur as frequently as daily.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
2.2.9 A verification schedule shall be prepared and implemented for each activity. The schedule shall include: - Verification activities - Frequency of completion - (Designated) person responsible for each activity - A log of each of the activities. Results of verification shall be recorded and communicated to the HACCP food safety team. Record keeping and documentation is sufficient to demonstrate the HACCP controls are established and effective.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
2.2.10 - All revisions to the HACCP Food Safety Plan are documented. Documentation includes a record of the reason for any changes or amendments to documents. - Changes to HACCP programs that impact customer products or are in conflict with existing specifications shall be approved by the customer prior to implementation.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
2.3 HACCP/Food Safety Plan Implementation and Execution
-
2.3.1 CCP monitoring and documentation follows the HACCP Food Safety plan. Gaps in records indicate the reason. CCP / Preventive Controls checks are evaluated for continuity. Review of records, where acceptable limits have not been achieved, corrective actions for issues found are on file. Any gaps in records indicate the reason (e.g. line down). CCP / Preventive controls checks are evaluated for continuity.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
2.3.2 HACCP / Food Safety records are present and include the date, time and result of the measurement. A Pre-shipment review/verification signature / intitals and date are present on all records. Where records are in electronic form, there shall be evidence that records have been checked and verified.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
2.3.3 HACCP Food Safety deviations are recorded and communicated daily. Documented corrective actions containing root cause analysis and verification exist.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
2.3.4 Corrective actions resulting in Holds, Recovery or other actions must have proper disposition and records. There shall be a procedure for handling and correcting failures identified in the food safety and quality management system. The corrective action records are reviewed and signed using initials or signature and dated by qualified management staff and analyzed for trends, to prevent reoccurrence. Completed root cause analysis and handling of preventive action shall be in evidence. The corrective action process shall include: -Clear documentation of the non-conformity -assessment of consequences by a suitably competent and authorised person -The corrective action to address the immediate issue -Completion of root cause analysis to identify the fundamental cause (root cause) of the non-conformity -Appropriate timescales for corrective and preventive actions -The person(s) responsible for corrective and preventive actions -Verification that the corrective and preventive actions have been implemented and are effective. Root cause analysis shall also be used to prevent recurrence of non-conformities and to implement ongoing improvements when analysis of non-conformities for trends shows there has been a significant increase in a type of non-conformity. The site shall ensure that any out-of-specification product is effectively managed to prevent unauthorised release.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
2.3.5 The Facility's HAACP and Food Safety Plans are audited by a third party at least once per year. If required by law or the customer, a HACCP certification for the facility is present or, if in the USA a HACCP PCQI certification. Records shall be present.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
3.0 Food Safety & Quality Systems
3.1 Food Safety and Quality Plan
-
3.1.1 All food safety and quality monitoring (not already documented in a HACCP Food Safety Plan) must have written procedures, which contain: -Person responsible -Frequency -Limits and corrective actions taken when those limits are exceeded. Monitoring must also be documented. Shall include: -Food Safety and Quality Policies (documentation that outlines the methods and procedures) -Processes and procedures comprising the Quality and Food Safety manual All polices and procedures must take account of and reference: -Customer requirements and expectations -Quality awareness with clear objectives -Applicable laws and regulations (as applicable in the country of manufacture and any countries exported to) -Clear identification of the person(s) responsible for approving changes to these documents Senior management has authorized and signed food safety and quality policies. The facility must be able to demonstrate that they have developed and maintained all documented procedures required for a food safety and quality management system. Documented procedures should show how the facility meets customer and local requirements. The facility must be able to show that they have access to laws and regulations by either having electronic access or maintaining a current list of laws/regulations.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
3.1.2 Each pre-requisite program / preventive controls must be validated, verified and must be signed off by the specific expert (SQF/QS-FS designee) indicating that the verification and validation have been completed. Frequency (at least annually) & methods used to validate food safety fundamentals are documented and meet their intended purpose. As a guide these may include the following, although this is not an exhaustive list: -Cleaning and sanitizing -Pest management -Maintenance programs for equipment and buildings -Personal hygiene requirements -Staff training -Supplier approval and purchasing or Supply Chain -Transportation arrangements, including receiving and shipping -Processes to prevent cross-contamination -Allergen controls management -Chemical control -Environmental monitoring program -Traceability / Recall -VACCP -TACCP -Good Agricultural Practices (where applicable),etc.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
3.1.3 The site shall have a documented procedure for handling and correcting failures identified in the FS & Quality system. Where a non-conformity places the safety, authenticity or legality of a product at risk, or where there is an adverse trend in quality, this shall be investigated and recorded including: -Clear documentation of the non-conformity -Assessment of consequences by a suitably competent and authorized person. Include: -The corrective action to address the immediate issue -Completion of root cause analysis to identify the fundamental cause (root cause) of the non-conformity -Appropriate timescales for corrections and corrective / preventive actions -The person(s) responsible for corrections and corrective / preventive actions -Verification that the corrections and corrective / preventive actions have been implemented and are effective. -A continuous improvement program shall be established for addressing failures. The program shall: - Define the process used for continuous improvement. - Define the measurement and tracking tools used. - Define goals and objectives. - Provide for performance to be measured against those goals.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
3.1.4 Product quality risk assessments shall be developed, effectively implemented and maintained in accordance with a risk-based method such as HACCP for all product categories produced by the supplier. The product quality risk assessment shall be independent from the food safety HACCP plan and must identify quality threats, key quality control points, and control measures used. The product quality risk assessments shall be developed and maintained by a multidisciplinary team that includes those facility staff with technical, production, engineering and product development knowledge of the relevant products and associated processes. The product quality risk assessments shall be reviewed following any significant changes to process, equipment or raw materials. Where no changes occur, the risk assessment shall be reviewed annually. The product quality risk assessments shall identify and outline key quality control points (QCP). Procedures shall include provisions for ensuring the production of the product is controlled. The supplier shall have an implemented procedure which shows: - The flow of products from point of material arrival through to shipping, using a flow chart or similar format. - A number, letter or similar designation for each step of the process that is used to control product quality as defined by the product quality risk assessment The steps identified on the process illustration shall be listed in a matrix or similar format and will include: - A reference for each key process stage to the appropriate standard operating procedure or work instruction used to control that stage - Method used to control each stage of the process identified as a QCP - Role responsible for controlling each stage identified as a QCP - Critical limits for each stage which will ensure specification limits are met - Corrective action to be taken when limits are not achieved - Records shall be maintained QCP's shall be routinely monitored during the production of product with records maintained.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
3.1.4.1 Supplier shall nominate customer specific Quality Teams if required who will drive quality management and improvement initiatives. Supplier shall nominate a cross functional Quality Team who, in addition to their normal roles, shall be responsible for coordinating product quality improvement initiatives. The quality team shall be at facility level and include functions who directly impact the production of product. The Quality team shall nominate a team leader. The leader shall not be the facility technical / quality assurance manager. The Quality Team shall be documented and have defined goals and objectives. Goals and objectives shall be reviewed at least annually. The Quality Team shall have action plans for goals and objectives which shall be subject to a quarterly senior management progress review. The Quality Team shall have a Management sponsor who has appropriate seniority to ensure quality improvement initiatives can be implemented. The Quality Team shall regularly review product quality and implement initiatives to drive continuous improvement. The Quality Team shall support in verifying the accuracy of the QAP against the production flow. A copy of the latest QAP, signed as accurate by the Quality Team leader shall be retained.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Preventive action -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
3.1.4.2 Key Performance Indicators (KPIs) shall be clearly defined for products. Supplier shall have documented, approved KPI's defined for all products unless otherwise approved in writing. KPI's shall be linked to the key steps of the process that define quality identified in the product quality risk assessment. Product KPI's defined shall be routinely monitored and analyzed. Product KPI's shall be monitored; with records kept at a frequency defined in the product specification. Records of KPI monitoring calibration shall be maintained and shall include: - Results of training sessions - Calibration records for all relevant monitoring equipment Production KPI data and analysis of the results shall be communicated to all levels at least monthly. Where KPI's are reported less frequently, the KPI's should be communicated each time. There is evidence that where appropriate Production KPI data is used to drive quality improvement.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action
-
Repeat -
3.2 Allergen Management
-
3.2.1 A written allergen management program is present and utilized. All allergens of interest, for the country where the facility is located are named. New & existing supplier verification for allergens is conducted. The program includes handling of raw materials, including: - receiving - color coding to designate allergens, including pre-weighing of allergen containing raw materials - dedicated storage bins & scoops for weighing, - WIP - rework - spills - cleanup - waste - tools - storage & segregation from non-allergen containing materials. The program includes compliance to specifications throughout the purchasing & supply chain, including the regulations in those countries where the product is to be shipped. A list of all allergen containing materials handled on-site, whether raw materials, intermediate products or finished products shall be present and maintained. Ingredients that are allergens are identified as such on all formulations, batch or raw material production records.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
3.2.2 The allergen program addresses food brought onto the site by staff, caterers specifying where allergen containing food is stored or stocked (snack machines). The program shall assure that traceability of rework & WIP is maintained. The program addresses: -The use of protective clothing while handling allergens -The use of identified, dedicated equipment and utensils for pre-weigh / kitting / processing / spills. -Updates to the program when new allergens & allergenic sources are brought into the facility. -Actions to take when there is an inadvertent event of cross-contamination. -Defines frequency of program review and reports exist -Allergen containing waste. The potential for cross-contamination at the raw material suppliers sites has been assessed & includes sensitivities. Allergen-specific training, including documentation, is provided to all employees who work with or handle allergens. Program addresses inadvertent cross-contamination during manufacture. The program shall include consideration of allergens in the Sanitary Design process.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
3.2.3 The program is verified & monitored regarding effectiveness & changed when & where necessary. Verification activities include -Incoming raw materials & subcomponents -Packaging & finished products -Manufacturing cross-contact -Label review -Label control for issuing, reconciliation from run to run, new & reprints. Results are documented.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat
-
3.2.4 A scheduling matrix has been developed and is used for scheduling production and sanitation activities. A procedure is established to maintain product quality during the startup and change over of operations and are documented. At a minimum, the procedures should include verification: -Ingredients -Allergen controls -Packaging material -Labels - pre-printed, batch printed, primary and secondary Changeovers: Removal of previous ingredients & verification, specifically addresses ensuring allergens are not transferred to a non-allergen containing product/other allergen products. -Sanitation -Change of ingredients -Packaging -Labeling. These shall include: - Start-up checklists that include verification of ingredients, packaging material, labeling with sign-off - A list of tasks for change-over that includes, removal of previous ingredients, allergen controls, sanitation, change of ingredients, packaging, and labeling, including any inconsistencies investigated and resolved. - Responsible parties for completing each task.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
3.2.5 A program is in place that includes packaging, identifies all allergens in products, including sub-components, for all markets and customers (including International, where applicable). The identification shall, as required by the customer, assess risk for all allergens & sensitivities. The risk assessment shall consider: - Consideration of the physical state of the allergenic material (i.e. powder, liquid, particulate) - Identification of potential points of cross-contamination (cross-contact) through the process flow - Assessment of the risk of allergen cross-contamination (cross-contact) at each process step - Identification of suitable controls to reduce or eliminate the risk of cross-contamination (cross-contact). There is a formal record of the risk assessment. Raw materials / ingredients, processing steps, processing aids, rework, manufacturing carryover are included in the assessment. Where claims are made regarding the suitability of a food for allergy or food sensitivity (where applicable), the claims shall be fully validated, being backed by documented data such as analysis of finished product, raw materials and post-cleaning swabs for specific allergens.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
3.2.6 Allergen testing including kits, PCR, visual, rinse water testing of CIP systems, external lab verification of presence/absence of allergens. Includes post Sanitation testing of lines and SSOPs that are specific to allergens, including dedicated cleaning equipment. An SSOP is present & in effect that addresses sanitation that occurs on an allergen containing line that is next to a non-allergen containing line. Frequency of testing shall be defined & backed by documented validation. CIP rinse water sample should be collected and tested for specific allergen on a weekly basis. Allergen testing should be performed randomly selected food contact surfaces on a weekly basis. Re-validation shall be done when process/product/ sanitation procedure changes occur. Limits of acceptable and unacceptable cleaning performance shall be defined for food contact surfaces and processing equipment. These limits shall be based on the potential hazards relevant to the product or processing area (e.g. microbiological, allergen, foreign-body contamination or product-to-product contamination). Therefore, acceptable levels of cleaning may be defined by visual appearance, ATP bioluminescence techniques (see glossary), microbiological testing, allergen testing or chemical testing as appropriate. Pre-Weight or Kitting Rooms are cleaned, inspected and swabbed on a frequency stated in procedures. High Risk Zone facilities: Production risk zones, including ambient- High-risk, high-care and ambient high-care production risk zones: Tested annually. - Layout, product flow and segregation - Building fabric - Maintenance - Staff facilities - Housekeeping and hygiene - Waste/waste disposal - Protective clothing
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
3.2.7 Ingredient statements must be verified to the: - raw material spec - ingredient disclosure or other - accompanying documents - including the COA for every raw material once every 12 months - when supplier is purchased by another company - when supplier is changed - as customer dictates. Where a justified, risk-based assessment demonstrates that the nature of the production process is such that: cross-contamination (cross-contact) from an allergen cannot be prevented, a warning should be included on the label. Legislation, national guidelines or codes of practice shall be used when making such a warning statement.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
3.2.8 Finished product labeling verification for product and packaging occurs for every product through Optiva or other electronic Product Life Cycle Management (PLM) system and anytime changes are made to the formula or packaging. There shall be effective process in place to ensure that labeling information is reviewed whenever changes occur to: - product recipe - raw materials - supplier of raw mats - country of origin of raw mats - legislation. Further label verification occurs at a minimum of every three years or as dictated by the customer.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
3.2.9 Non-conformities relating to allergen control shall be included specifically in the management meetings. Complaints or issues, whether internal or external shall be discussed. The company shall have procedures in place to keep abreast of legislation or scientific information relevant to allergen containing materials (such as protein presence in highly refined soybean containing materials).
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
3.3 Environmental Monitoring Program
-
3.3.1 Zoning: the company shall assure that the process flow from receiving through finished product shall be arranged to minimize the risk of product contamination. Zones shall be defined and identified on a facility map. Establish mapping (Zone map) -Supplier shall have a clearly communicated microbiological monitoring and testing policy that states the facilities intentions to meet obligations to produce safe and legal products. The micro policy shall be: -Supplier shall have a microbiological and analytical monitoring and testing policy/procedure -Signed by the senior leader (s) -Available in languages appropriate to the staff -Communicated to all appropriate staff
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
3.3.2 A documented Environmental Monitoring Program is based on risk, present & includes requirements for a map and master list of sampling locations for each production line to allow tracking of trends, zone requirements and risk assessment for establishing test frequencies. At a minimum the program should include: - sampling procedures - identification of sample locations - frequency of tests - target organism(s) (e.g. pathogens, spoilage organisms and/or indicator organisms) - test methods (e.g. settle plates, rapid testing and swabs) - recording and evaluation of results. - air plates and compressed air is tested for lactic acid. The program and its associated procedures shall be documented. The facility shall actively look for potential areas / niches / harborage areas where the target microorganism is likely to be found and then aggressively swab this area. If a target microorganism is found, vector swabbing must be done, and additional cleaning and sanitation must be done. The area must be retested three times to obtain a negative. Root cause for the positive detection must be established.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
3.3.3 The program requirements include: - frequency of testing - limits sampling procedures - guidance for retesting - which organisms are tested - methods used & where; i.e. at company microbiology lab, outside laboratory, etc. - the actions to take when trends indicate unacceptable results (corrective actions) and - when in the process testing is to occur. The program is to include food and non-food contact surfaces. Microbiological limits for acceptable and unacceptable cleaning performance shall be defined for high-risk / high-care production risk zones. Test results shall be recorded and reviewed on a regular basis to identify trends. The GENE-UP BACTVIAB PMAxx or EnviroPro with PMAxx methods (or equivalent) are recommended to ensure that false positive results are not acquired due to the test detecting free DNA/dead cells. The method used must be validated for the specific buffer/broth used as a neutralizing solution with the sponge swab.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
3.3.4 The program is to include that results & trends are routinely communicated to & discussed with senior management. The significance of external lab results shall be understood & acted upon accordingly.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
3.3.5 Refrigeration evaporators (such as coils, fans, inside drip pans, rubber-like cooler door seals, etc.) and areas in coolers shall be included in the Environmental Monitoring Program site list.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
4.0 Food Safety Process Control
4.1 Microbiology
-
4.1.1 There is a documented Pre-Operational Swabbing /Bioluminescence Testing & Inspection program to verify effectiveness and line conditions of cleaning prior to startup of production. - Roles, personnel requirements & responsibilities are present: - Full time employee. - Trained employee. - Employee not performing cleaning / sanitation. - Tools to be used are listed with specifics to flashlights and mirrors. - The areas to be inspected are listed, especially hard to reach / inspect areas. - Access items to reach hard to reach / inspect area is listed. - Safety and LOTO requirements are stated. - The cleanliness of equipment shall be visually checked / verified and documented before swabbing or equipment is released back into production, based off zone. - Other pre-requisite programs are being followed while performing inspections and swabbing. - Foreign material contamination risk review / inspection adequately performed before the line is released. - Swab results are documented with documentation of failures and rechecks. - Records of findings are present and corrective actions are present. - Records to be reviewed and/or verified by a Lead or Supervisor. - Training for inspection personnel is stated and documented. Microbiological limits for acceptable and unacceptable cleaning performance shall be defined for high-risk / high-care production risk zones. Non-food contact surfaces in the area of the operation are to be included in the program including: - Ceilings - Packaging - Supplies in the area - Walls - Floors - Drains, etc. Effective cleaning practices are evident.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.1.1.1a Pre-Operational Inspections are verified weekly by a trained member of the Quality department. - Inspections to be documented. - Notations of non-conformances documented. - Corrective actions documented. - Documentation of re-cleaning and re-inspection. - Verification needs to focus on the inspectors addressing pre-op conditions Pre-Operational Inspections are verified quarterly by Quality Assurance Management. - Inspections to be documented. - Notations of non-conformances documented. - Corrective actions documented. - Documentation of re-cleaning and re-inspection.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.1.1.1b ATP Swabbing failure process followed: - First fail = retest - Second fail = Letheen swab taken for micro - Re-clean / CIP prior to releasing to production
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventiv action -
-
Repeat -
-
4.1.1.2 Pre-Operational Inspections to be performed by Quality Assurance management at a minimum of quarterly. These inspections are to include: - Documentation of inspection. - Documentation of non-conformances. - Documentation of corrective actions take. - Documentation of re-cleaning, re-inspection and/or re-swabbing.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.1.1.3 Compliance to the following requirements reviewed prior to releasing the line for production: - Hand wash station(s) have soap, sanitizer, paper towel (or a functioning hand dryer) and hot water - All packaging materials from previous run must be removed. - No uncapped lines/ports - Foreign material - Temporary repairs - GMP's - Disassembling of components to ensure effective cleaning and sanitizing - No condensation above food contact zones/product level - All drains shall be flowing and unblocked, no stagnant water - No equipment parts, clamps, hoses, gaskets in the hand wash sinks or on the floor - No foul odors present
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.1.2 Written record of the Pre-Operational inspection and swabbing records include: - Notations when non-conformances are found - Corrective actions - Re-inspection / re-swabbing of the area. The cleanliness of equipment shall be checked before equipment is released back into production. Swab results are documented with documentation of failures and rechecks. Verification of corrective actions is performed prior to releasing back into production. Records are present of findings to be reviewed and/or verified by a Lead or Supervisor, including: - Visual - Analytical - Microbiological checks - Corrective actions The results including failures are used to identify trends in cleaning performance and to investigate improvements where required. Non-food contact surfaces in the Operations areas are to be included in the program.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.1.3 Lab coats that are worn in the micro lab are not worn anywhere else in the facility.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.1.4 No pathogen testing is performed within the facility unless required by governmental regulations. Pathogen testing (including pathogens tested as part of the site environmental monitoring program) shall be subcontracted to an external laboratory or, where conducted internally, the laboratory facility shall be fully segregated from the production and storage areas and have operating procedures to prevent any risk of product contamination of products or production areas. Pathogen & spoilage organism testing is performed per customer's / governmental requirements. - Duplicate samples are pulled when customer/governmental agency, etc. removes samples from facility. Facility must demonstrate that sampling for product for inspection or analysis is completed using recognized sampling methods. Testing for indicator organisms, as appropriate (APC, Coliform, E coli) has been established. Investigations and corrective action plans are documented for out-of-spec conditions. PRODUCE: Suppliers are expected to develop a finished product pathogen testing program to verify that pathogens do not exceed the limits for the food matrix, established by the applicable regulatory authority. The supplier is responsible for understanding the environmental risk, the inherent risk of the pathogen in the food matrix and the applicable regulatory requirements when establishing their pathogen testing protocols. When testing for pathogens, McDonald’s expects a test and hold procedure to be established that is recognized and supported by the applicable food safety authority. When test and hold is not possible, process used should be aligned with applicable regulation and authorities. At a minimum McDonald’s expects finished product testing that includes Listeria Monocytogenes that meets the applicable regulatory requirements. Where multiple product types (e.g.: shredded lettuce, slivered onions, salad mix, etc.) are produced, spread out monthly sampling events over time. Testing must be conducted by credible analytical laboratories (including supplier’s own lab) using approved, published, official, validated methods; these methods shall be recognized by the local regulatory agencies. Where possible, the laboratories must be accredited for these analyses to an international standard (or a recognized national standard) and/or participating in a proficiency testing program.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.1.5 Microbiologist is certified or degreed in microbiology. Proficiency Testing and training is conducted/updated once per year. A qualified individual may be a microbiologist, trained in microbiological practices or equivalent through experience. At a minimum, the person performing microbiological testing undergoes competency testing by an outside recognized lab (check sample testing).
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat
-
4.1.6 Analytical & Micro: Outside lab is certified for all tests that are performed by the plant. Proof of certification is present to show that the laboratory is authorized to conduct specific tests. Laboratories and testing methods are licensed/approved with documented training of lab personnel.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.1.7 Facility must demonstrate that sampling for product for inspection or analysis is completed using recognized sampling methods. There shall be a scheduled program of product testing which may include: - Microbiological - Chemical - Physical - Organoleptic testing according to risk The methods, including: - Processes for obtaining product samples (including where appropriate, their delivery to a laboratory) - Frequency - Specified limits shall be documented. Microbiological and analytical testing for dairy products is conducted as required.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action-
-
Preventive action -
-
Repeat -
-
4.1.8 Analytical & Micro: A written contingency (backup) plan is present for equipment that does not function properly. Procedures are established for the calibration and accuracy of "key" measurement equipment and testing equipment for: - Labs - QC - Manufacturing process, including schedules and reference standards. Repairs and equipment modifications are professionally completed without the use of: - String - Tape - Wirer - Other improvised materials and in a manner to prevent potential contamination.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.1.9 Product that is tested for pathogens is segregated and in a "lock-down" mode (cannot be shipped). Effective Test and Hold program in place. A full cleaning and sanitizing cycle to be performed for all equipment and food contact utensils / tools while manufacturing the tested lot(s). Product matrices shall be reviewed and update when new pathogen testing is added. Rework cannot be added to product lots that are tested for pathogens. Pre-shipment reviews shall not be considered complete until all pathogen / adulterant testing results ae reviewed and confirmed acceptable. All pathogen / adulterant testing results must be reviewed by a member of Quality Systems. Product that has tested presumptive positive for a pathogen has had the inventory count verified. Product that has tested positive for a pathogen is counted at least weekly until disposition is made. YUM! Finished product, for the required Yum products, must be tested for Salmonella and E coli at a frequency specified by Yum! QA (or other testing method as defined in Yum! commodity specific testing requirements.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
4.2 Analytical
-
4.2.1 Testing equipment is clean and well maintained. Lab is clean and free of clutter. No food, beverages or personal items are stored in the laboratory. All chemicals are properly labeled (e.g. chemical name, lot number, day received, day opened). Labs are physically separated from processing areas and restricted access signage is present. Lab shall be located, designed and operated to eliminate potential risks to product safety. Controls shall be documented, implemented and include consideration of: - operating procedures to contain laboratory activities, including the design and operation of drainage and ventilation systems. - access and security of the facility. - movement of laboratory personnel. - hygiene and protective clothing arrangements. - movement of materials that may pose a risk to products, raw materials or the production area, into and out of the laboratory, including the disposal of laboratory waste. - the management and monitoring of laboratory equipment. Where testing activities are performed in production or storage areas (e.g. at the line tests or rapid tests) these shall be located, designed or operated to prevent product contamination.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.2.2 All test methods shall be documented and be based on recognized, official test methods. Procedures for testing are documented. Test frequencies shall be specified and the laboratory methods validated for accuracy and reproducibility. End product testing is conducted. Testing areas are designed so that a separation is present so that there will not be a detrimental effect on manufacturing. Critical limits and testing methodologies for food safety critical equipment shall be based on risk assessment unless otherwise defined.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.2.3 All employees executing analytical testing are to be calibrated to a single appropriate standard of working. Lab participates in outside competency testing (accuracy and precision). Lab is accredited. If so, by what body? Testing includes batching, processing and packaging areas of manufacturing. Training / retraining is conducted once per year. Training / retraining is documented. Split samples are sent to an outside qualified lab twice per year OR lab belongs to internal competency check program. List the 3rd party used for proficiency / competency testing.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.2.4 The facility has a finished goods inspection program to ensure conformance to internal or customer specifications. Review of analytical testing records confirm results conform to specifications. Inspection records are reviewed before finished goods are released for delivery. A 'near miss' reporting system for food safety non-conformance must be in-place and linked to a review, action process and communication plan. The site shall track 'near misses' and they are used as to bring the process into control. There shall be a microbiological and analytical testing policy / procedure.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.2.5 Documented procedures are in place for reviewing and accepting all customers product specifications and related testing methods. McD's SQMS: Applicable to all formulated products and bakery suppliers: - Palm oil users: compliance with McDonald's Land Management Policy pertaining to Roundtable on Sustainable Palm Oil (RSPO) membership and certification according McDonald’s Palm oil policy - Compliance to McDonald’s oil product specification
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.2.6 A process is in place to verify: - In-house capability - Sampling plan - Required test methods - Equipment and - Analyst training to ensure conformance with all customers specifications. This may be part of the in-house internal audit program.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.2.7 Specification Compliance Report based on format agreed with each customer is available, where specified, and is submitted to each customer, as required, at the agreed frequency. There is a process to monitor specification compliance and corrective actions taken. Test results are presented graphically so that trends may be noted.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.2.8 All raw materials, shall have written specifications, including packaging, hazardous chemicals & processing aids). Appropriate processes and procedures shall be established and implemented to verify the consistency of the raw materials according to the specifications. An effective process is in place to verify compliance of the materials with the respective specifications prior to their use in the manufacturing of the customer's products. Must be documented, current & comply with relevant legislation. Specifications must be current and include defined limits which may affect quality or safety of the final products ((e.g. chemical, microbiological, physical or allergen standards). Guidance: All raw material, ingredients and primary packaging (packaging that comes in direct contact with the product) specifications have been communicated with suppliers/vendors of these raw materials, ingredients and primary packaging. BEEF: Applicable to raw material suppliers of beef: - Verification of supplier on Approved Supplier List - Evidence of a “pass” for BSE Firewall and animal health and welfare audits - Evidence of a score of ≥ 75% for GMP/HACCP audit or GFSI certification - Evidence of passing Halal/Kosher audit, by an authority recognized by the country of origin and consumption where applicable - Compliance with VAS - If using an on-site laboratory performing e Coli O157:H7 testing – must meet 3rd party annual audit requirement, certified by an external accredited laboratory and a COA submitted with each raw material lot where required - Laboratory data and trend analysis confirm microbiological criteria (APC/TPC, E coli, etc.) meet local regulatory requirements and McDonald’s specifications FRESH PRODUCE: - Supplier shall have documented approved raw material sourcing (e.g. farms, packing house) - Supplier shall be able to demonstrate that raw material sourcing is in compliance with Good Agriculture Practice (GAP) - Global G.A.P., Global G.A.P. Risk Assessment on Social Practice (GRASP) plus McD’s addendum. Formulated Products: Supplier shall be able to demonstrate that raw material sourcing is in compliance with a Good Agriculture Practice (GAP) scheme that has been benchmarked to Sustainable Agriculture Initiative (SAI) platform Farm Sustainability Assessment (FSA) for all crops.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
4.3 Process Monitoring
-
4.3.1 Appropriate monitoring is performed by trained personnel. Includes daily, weekly, monthly, on some set schedule, including batching, pre-weigh, etc. Monitoring is performed according to a published schedule. Monitoring addresses all quality attributes. When there is a loss of control, the measurement or monitoring activity is able to detect this. Frequency & quality of samples is adequate to detect loss of control.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.3.2 Pasteurization/cooking temperatures are monitored and recorded. Monitoring is performed according to a published schedule and by trained personnel. When there is a loss of control, the measurement or monitoring activity is able to detect this. Frequency & quality of samples is adequate to detect loss of control.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.3.3 Process specifications shall be in accordance with the agreed customer finished product specification. Monitoring records state process parameter and target.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.3.4 Records of checks shall be retained for frequency & methodology. Documented procedures shall be in place to ensure that products are packed into correct packaging & correctly labeled. Procedures shall be in place to ensure that all products are packed into the correct packaging and correctly labelled. These shall include checks: - at the start of packing - during the packing run (e.g. at predefined intervals and when printed packaging or labels are brought to the line during the production run) - when changing batches of packaging materials - at the end of each production run. When changing batches of packaging materials, the checks shall also include verification of any printing carried out at the packing state, including: Date coding, batch coding, quantity indication, bar coding, Country of Origin, as applicable. The verification of film requires 2 signatures for every change. The checks shall also include verification of any printing carried out at the packing stage including, as appropriate: - date coding - batch coding - quantity indication - pricing information - bar coding - country of origin - allergen information.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.3.5 Employees are sufficiently knowledgeable to be able to carry out corrective actions so that control of the process is regained. There are documented process procedures available (batch cards, Pre-weigh, etc.) for every process. Associated documents are reviewed by designated officials of the company. Frequency is to be determined by company or customer. Procedures are in place to re-establish control & to determine the fate of the affected product. Process audits are documented and measured for alignment to written specification from customer, corporate, internal criteria. Results are communicated to appropriate associates. Each facility shall establish the parameters within which the production line is expected to operate. Process control and monitoring activities shall be established to document the plant’s ability to produce products within the established parameters. A formal system exists for the recording and resolution of corrective actions.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.3.6 Processing parameters or in-process measurements shall be established, validated, and verified at a determined frequency to meet all appropriate requirements. Adequate statistical process control shall be implemented where applicable to improve product consistency, reduce process variation and improve overall capability of the process.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.3.7 There shall be a documented procedure on handling rework (also may be called WIP, Work-In-Progress), which may be reintroduced into the process immediately or at a later time. This procedure shall include: - Means of ensuring proper traceability - Allowed amount of rework per batch - Allergen / sensitive ingredients considerations (e.g. "same into same" or "like into like") - Addition methods - Any products that are forbidden to be reintroduced (specifically, returned product) - Allowable shelf-life prior to reintroduction, and - Any other special handling instructions. There must be a record of rework when it is reintroduced back into the process. There shall be no rework from products held for microbiological failures without the specific permission of Global FS, QS and Regulatory.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.3.8 Where process parameters or product quality are controlled by line-monitoring devices these shall be linked to a suitable failure alert that is routinely tested.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.3.9 Visible Process: Real time control chart is used at all identified locations on processing lines for all products requiring SPC. The operator keeps the process at target, and only makes necessary and effective adjustments when the process is OUT OF CONTROL AS INDICATED BY THE CHART. YUM! QSA: Implementing statistical methods into production process will include: - Statistical process control integrated into the processing procedures and practiced by production personnel at a minimum for all KPIs identified on the Yum! Brands KPI reports, Quality Assurance Programs, or Specification. - Control charts have defined upper and lower control limits, not specification limits. - Operating personnel demonstrate fundamental understanding of statistical control. - Documented procedures for the use of charts and how to identify and respond to "special causes". - SPC rules should be documented and personnel trained. - Production personnel appropriately respond to out-of-control circumstances or decision rules for control charts. Control Charts should be in the area where measurements are taken, and updated when the tests are completed. QC can conduct the actual charting, but production personnel must understand and react appropriately to "out of control" processes. Confirmation of the proper use of control charts may be made through observation. Simply having control charts does not support an automatic award of points. For batch systems it may not be used for real time process control but must be used for long term continuous improvement.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.3.10 Capable Process - All processes are capable as determined by all five criteria below have been met at all process locations where there is an applicable specification: 1. The analysis is based upon a minimum of 150 data points. 2. Real time control charting is being effectively used to control this point in the process. 3.There are no unexplained out of control conditions. 4. The data fits a normal curve, and 5. 99.97% of the process output falls within the specification limits. More specifically, the spec limit that is closest to the process average, minus the process average, when divided by three standard deviations equals at least 1. If less than 1 the process is not capable (CpK determination).
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
4.3.11 Optimized Process - A process is in place to update the process sigmas and optimize the targets on the real time control charts. Statistical data is used to manage process improvement and optimize processes. Increased efficiency, reduction of waste, increased product quality and consistency, and cost savings are evident as the result of the process control implementation. Weight Control is present
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
5.0 Sanitation Program
5.1 General Facility/ Housekeeping
-
5.1.1 Added high touch areas All housekeeping cleaning activities are documented and located on a Master Cleaning Schedule. This schedule lists all equipment, utensils and areas that require cleaning and sanitizing, and their cleaning frequency. The master cleaning schedule is internally audited on a regular basis, at a minimum, monthly basis, to verify compliance. • This includes non-food contact equipment, interior / exterior, overhead piping, lights & light fixtures, storage racks, structure, ceilings, walls, floors, air ducts and vents, hand trucks, forklifts, etc. The schedule identifies the frequency of cleaning. Procedures & records exist for all tasks. • Where hand dip stations and/or foot baths are used, they must be cleaned and free of particulates, dirt and debris and changed often enough with potable water at the appropriate temperature to keep the sanitizer concentration in the acceptable range. • For non-disposable items (e.g., gloves/aprons/hard hats/PPE) the condition and frequency for the cleaning and changing shall be established. • Trash receptacles are emptied frequently to prevent overflow and kept in sanitary condition with no offensive odors. • Identified high touch areas or areas of concern shall be added to the Master Cleaning schedule.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
5.1.2 All employee areas such as restrooms, wash areas, change rooms and lunch rooms / canteens (inside and outside) are cleaned on a scheduled frequency with records of completion. - Change rooms and toilets are segregated from the production area. - These facilities are clean, orderly and well lit. - Exhaust is vented to outside. - Lockers suitably designed to disallow inappropriate use (e.g., storage of items on top). - Work clothes and street clothes appropriately stored to prevent cross-contamination. - Verification of the records and inspection is evident.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
5.1.3 General areas (e. g., warehouse, aisles, battery charging maintenance shop & satellite locations, chemical cages) are clean and in good repair. Standing water or evidence of aged spillage are not evident on the floors. Walls, ceilings and overheads inside and outside production areas are clean without condensate. Drains all function properly.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
5.1.4 Chemicals and equipment are suitable for use in a food manufacturing establishment or stored in a secured area outside of processing/manufacturing areas. In-use food process sanitation equipment should not be stored with Housekeeping. Color coding, or labeling may be used. Restroom cleaning utensils are separated and identified. When chemicals will not fit into designated storage there shall be a written risk assessment to determine the level of risk. Color coding, or labeling may be used. Restroom cleaning utensils are separated and identified. When chemicals will not fit into designated storage there shall be a written risk assessment to determine the level of risk. Additonal expectations include: - an approved list of chemicals for purchase - availability of material safety data sheets and specifications - confirmation of suitability for use in a food-processing environment - avoidance of strongly scented products - the labelling and/or identification of containers of chemicals at all times - a designated storage area (separate from chemicals used as raw materials in products) with restricted access to authorized personnel - use by trained personnel only. - procedures to manage any spills - procedures for the safe, legal disposal or return, of obsolete or out-of-date chemicals and empty chemical containers
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
5.1.5 Restrooms and washrooms have adequate supplies. Restroom fixtures work properly, no offensive odors, trash receptacles are covered. Water is hot at the hand wash stations. - There are hand wash reminder signs placed at the entrance to the facility and at all hand wash stations. They are operational and provided with hot / warm water at 38 °C / 100 °F minimum, where appropriate. Such hand-washing facilities shall provide, at a minimum: - Advisory signs to prompt handwashing - A sufficient quantity of water at a suitable temperature - Water taps with hands-free operation - Liquid / foam soap - Single-use towels or suitably designed and located air driers.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat
-
Corrective action
-
Preventive action
-
Repeat
-
Corrective action
-
Preventive action
-
Repeat -
-
5.1.6 Freezers do not have aged ice build-up. No mold or mildew present in coolers. Products and area temperatures at receiving, storage and loading are monitored and documented and meet required specifications.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
5.2 Processing Sanitation Program
-
5.2.1 Added High Touch A documented Master Cleaning Schedule is in place for all tasks other than daily. Identified high touch areas or areas of concern shall be added to the Master Cleaning schedule. This includes inside and outside the plant: - e.g., bulk tanks - processing equipment - packaging equipment - bins (such as kitting of bulk ingredients) - structure - doors - floors - lights - storage areas - staff amenities - toilet facilities - drains: a schematic drain map is present for each operational room and used to document and verify drain cleaning. The schedule identifies the frequency of cleaning and shall address cleaning between breaks. Procedures exist for all tasks and frequency is based on risk. Assessment shall include risk from cleaning chemical residues on food contact surfaces. The detailed schedule shall be internally audited periodically for its maintenance and effectiveness. The facility sanitation plan will be effectively implemented and routinely verified including maintenance for food safety. McD's SQMS: There is no evidence of significant build-up present during production. Excess moisture, standing water and condensation are removed from equipment and the environment prior to start of operations. Tools such as knives, saws, trimmers and others used in processing are adequately cleaned and sanitized during processing. A process is in place to ensure suitability of use of any piece of equipment that has not been used for over 4 hours after last sanitation, based on risk assessment.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
5.2.2 Procedures are present and specific to the site exist for all tasks to include: Frequency of cleaning, based on risk. - organisms of concern - person responsible - area to be cleaned & include detailed procedure steps - equipment & utensils, including dismantling equipment for cleaning purposes where required - cleaning chemicals - concentrations of dilution - water temperature - Records to be kept - Verification / Inspection / effectiveness - Safety / precautionary notes - Include CIP & COP systems & each task involved. - Visual inspection verifying valve movement Management representative shall review records for completeness.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
5.2.3 Brushes & Equipment are color coded to indicate usage. • Food • non-food • drains, • house-keeping utensils are identified. The color coding is posted and is dedicated for use in an area or to a specific purpose.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
5.2.4 A procedure exists for verification and Validation of the CIP and COP systems. New CIP Systems are validated for proper cleaning function before they are commissioned and after changes and monitored per the cleaning program to ensure they are working properly. Master flow meter calibrations and validations performed at a minimum of annually. CIP cleaned circuits are validated annually. The CIP System temperature charts are verified and signed daily. - Chemical concentrations / detergent concentrations are checked for cleaners and sanitizers daily for CIP & COP systems. - Velocity and temperature are verified at a defined frequency. - Filters, where fitted, shall be cleaned and inspected at a defined frequency Where used, flexible hoses shall be stored hygienically when not in use, and inspected at a defined frequency to ensure that they are in good condition. CIP facilities, where used, shall be monitored at a defined frequency based on risk. This may include: - Ensuring correct connections, piping and settings are in place - Confirming the process is operating correctly (e.g. valves opening/closing sequentially, spray balls are operating correctly) - Ensuring effective completion of the cleaning cycle - Monitoring for effective results, including draining where required. Procedures shall define the action to be taken if monitoring indicates that processing is outside the defined limits. Design includes no potential cross contamination risks.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
5.2.4.1 If CIP systems are controlled by PLC systems; the addition, modification or removal of set points are to be restricted to Management and above. Set points are defined as, but not limited to: - Time - Temperature - Concentration - Flow
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat
-
Corrective action -
-
Preventive action -
-
Repeat -
-
5.2.4.2 The CIP systems electronic chart record system should document the following: - Temperatures - Flow rates in gallons per minute or per square inch - Conductivity or chemical concentration - Each CIP cleaning steps' total time
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat
-
Corrective action -
-
Preventive action -
-
Repeat -
-
5.2.5 - A schematic diagram of the layout of the CIP system including process piping circuits shall be available. - An inspection report or other validation that shows scavenge/return pumps are operated to ensure there is no buildup of CIP solutions. - Recovered post-rinse solutions shall be monitored for build-up of carry-over from the detergent tanks. - Spray balls / rotating spray devices provide full coverage. - CIP equipment has adequate separation from active product lines, including make or break connections with proxy switches as interlocks; to prevent or safeguard against cross-contamination. - CIP equipment shall include detergent tanks are kept stocked up and a log maintained of when these are drain cleaned filled and emptied.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
5.2.6 All reports are present for in-house inspection, third party audits verifications, including those from chemical companies, regulators and contractor activity. Findings have corrective actions and are acted upon.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
5.2.7 Cleaning is verified. Cleaning verifications such as Swabs and/or ATP on CIP cleaned surfaces that include (when applicable): - Vessel /tank interior - Vessel / tank components attached - Line associated PD pumps - Line associated fillers Pre-op findings are trended & corrective actions are verified. Limits for acceptable and unacceptable cleaning performance shall be defined for high-risk/high-care production risk zones. Acceptable levels of cleaning shall be defined, for example, by: - Visual appearance - ATP bioluminescence techniques - Microbiological testing or - Chemical testing as appropriate. Deficiencies are addressed and records are maintained before production start-up and corrective actions are documented. These may include: - Process steps and/or microbiological interventions - Implemented to minimize or eliminate a microbiological hazard shall be: - validated - monitored - verified.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
5.3 Process Sanitation Implementation
-
5.3.1 Cleaning assignments are based on skill qualifications with documented training and skills assessment for each task. Skills assessed yearly. - Sanitation procedure/SOPs used to conduct the training - On-the-job training/other methods of training available for each employee, when applicable - Training is completed annually and records are maintained
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
5.3.2 Sanitation equipment is in sound condition and properly stored when not in use. - All cleaning chemicals and equipment are properly cleaned and stored in a secured area away from unauthorized use. - Cleaning chemicals and sanitizers may be in processing/production areas if they are secured and do not pose a risk of product contamination (e.g., sanitizer bottles, sanitizers on a dispensing unit, entrance sanitizer foam and hand dip stations). - Equipment is designed for effective sanitation and sanitation impact is considered when any upgrades or changes are made to equipment.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
5.3.3 Product labels and MSDS / SDS for all cleaners and sanitizers are available. Cleaners and sanitizers application and concentrations comply with the Sanitation Program and manufacturer's guidance.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
5.3.4 Records exist for all daily and periodic tasks completed. - Each task is and signed by the individual sanitation worker. - Verification and effectiveness (who is responsible) by an assigned person (manager, supervisor, etc.) is present on the records. - Includes CIP and COP Chart recording system. - Flow meter calibrations and verifications - Includes chemical testing kits for detergents and sanitizer concentrations. - Precautionary and/or safety notes are recorded, where necessary - Records to be kept, including records for completion and sign-off
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat
-
Corrective action
-
Preventive action
-
Repeat
-
Corrective action
-
Preventive action
-
Repeat -
-
5.3.5 Chemical concentrations are checked for cleaners and sanitizers daily from: - The dispensing systems - Any hand mixed solutions such as: - a foot bath - COP Tank - hand dip etc. They will be recorded daily, dated, signed and verified.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
5.3.6 Each facility shall have a written procedure that outlines the equipment teardowns which include who is responsible for each task. The facility shall perform extensive plant equipment self-inspections at least two times a year. This should include disassembling equipment and swabbing. This inspection is designed to be very thorough and detailed – beyond normal pre-op. This inspection is typically conducted on the weekend, after the “final cleanup”. Equipment should be inspected for: - Dead ends - “Ts” in the piping - Rough welds - Valves that do not pulse during the C.I.P. cycle - Improper drainage - Worn gaskets - Rubber impellers and other equipment made of materials that can wear or deteriorate over time - Including and other conditions that may result in inadequate cleaning. All CIP'd equipment (pumps, valves, gaskets, CIP skids, transfer line circuits, air blowing assemblies, etc. should be torn down. Engineering support provided where access within equipment is required for cleaning. Equipment should be accessible for cleaning. Modifications should be made where possible to facilitate better cleaning.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
5.3.7 Each facility shall have a program in place for dismantling, inspecting, cleaning and sanitizing all product contact gaskets and clamps. The frequency should be based on risk assessment and take into account the physical stresses placed on the gasket material as well as any periodic chemical stress which may cause etching of the surfaces. At a minimum, all gaskets shall be dismantled, inspected and cleaned at each allergen changeover (if applicable). Any gaskets showing evidence of damages, cracking, pitting, or etching of the surface shall be replaced. The program should have in place food contact gasket replacements and inspections. All gaskets shall be replaced every 6 months at a minimum. Higher frequency may be appropriate based on risk assessment.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
5.3.8 Sanitation and production are separated to prevent any possible contamination to products. This includes CIP circuits, over spray from hoses, etc. For ambient high-risk areas exist, a written risk assessment is present and it determines that risk of cross-contamination with pathogens. Also taken into account: - The raw materials and products - The flow of raw materials, packaging, products, equipment, personnel and waste - Air flow and quality - The provision and location of utilities (including drains).
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
5.3.9 Sanitation workers follow GMP to include: - hose nozzles. - food contact parts off the floor. Lubricants and solutions are clean and food grade.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
5.4 Sanitation for Maintenance
-
5.4.1 A maintenance-related food safety program is established. Records are maintained. The program reqires tool and part reconciliation as part of the process. Maintenance, engineering workers potential for cross contamination is also considered.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
5.4.2 The program includes: A procedure for notification of production & sanitation procedures occur before equipment is placed back into service following maintenance - Record of the notification of sanitation personnel (may be contained in sanitation reports) - Procedure for reconciling parts & tools. - When equipment is removed from high-risk or high-care area there shall be a procedure to verify the cleanliness and removal of contamination hazards before the equipment is accepted back into the area.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
5.4.3 Records are present that document that parts & tools are reconciled & food contact zones have been cleaned, sanitized and inspected (see 4.1.1) before release to production. - Missing parts & tools shall be documented & immediately brought to the attention of management. - Records of acceptance shall be maintained. - Appropriate sign-off by maintenance, sanitation, QA, etc. to ensure that cleaning and sanitation is completed following maintenance / emergency repairs where appropriate and documented.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
5.4.4 A procedure to undertake post maintenance cleaning and sanitation is implemented Food Safety or legality of products are not compromised during maintenance and cleaning operations. Where applicable, a purge process is performed after emergency repairs. (See 5.4.1) Where possible, dedicated tools and equipment shall be dedicated for use in that area and retained in same. Sanitation is completed following maintenance / emergency repairs where appropriate and documented.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
6.0 Grounds and Facility
6.1 Structure
-
6.1.1 Exterior walls, windows, screens, doors, docks, dock levelers and roofs are clean and in good condition. - Dock and personnel doors are properly sealed. - All vents, windows, dock doors that are kept open shall be screened. Screens shall be intact and opening of a size so as not to allow flying insects to enter. - Loading/unloading areas shall have dock shrouds that effectively prevent entrance of birds, pests, flying insects.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.1.2 The grounds, facility and entrance/egress points shall be designed so that dust and/or debris from the outside are not easily entering the facility. - Road or transport surfaces are suitably surfaced and maintained. - No weeds, tall grass, or idle equipment stored within the immediate vicinity (20 feet / 6 meters) of the building. - Trees shall be appropriately trimmed to eliminate access by rodents/animals.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.1.3 There is a Preventive Maintenance Program for the building including all ventilation, refrigeration, compressed air and other utilities. There is a reconciliation program to insure completion of tasks. - Mobile equipment is included in the program. - The maintenance requirements shall be defined when commissioning new equipment. - The inspection and maintenance of air handling units or HVAC systems must be part of the plant's planned maintenance activities These systems should operate properly as per the design criteria based on the current level of space and occupancy at the facility. The facility should assess these air systems with a mindset of promoting protective ventilation practices and intervention approach. This should include assessing the potential for: - increased air exchange - rebalancing of air movement - improvement of air filtration if necessary - proper inspection maintenance - cleaning - sanitation and disinfection of - housing - ducts - filters - grates for cleaning and sanitation. Grates should have no buildup. The person responsible for maintenance and inspection should be qualified to inspect HVAC and/or to have credentials to maintain and repair air handling units. - For Sensitive areas, there is positive air pressure. A HEPA filter is installed and functional in the ‘’ready-to-eat" high care areas. Air is filtered before entering the room. Air filtering units are regularly serviced and monitored. Factory is climate controlled as necessary for food safety and quality.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.1.4 Interior non-processing area: - Walls - Ceilings - Light covers - Floors and - Structures are clean and maintained in good repair - Equipment, structure, overhead vents shall not exhibit visible rust or flaking paint/rust. - All air outlets and filters are clean and there is adequate ventilation - Filters are changed to the PM or designated maintenance program Where portable wall structures are used as part of a high-risk area procedures shall be in place to ensure: - removeable walls are tight fitting - use is controlled, movement of the wall is only completed by trained and authorized staff - cleaning and reconditioning procedures are in place and completed prior to production. No condition exists which could deposit airborne contaminants.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.1.5 Facility layout, product flow and segregation is adequate to prevent contamination of the product. This includes: - forklift battery charging stations - employee movement between: - High and Low risk areas - Drains - Allergens - Items with specific identity preservation requirements, e.g., Kosher, Halal, Organic Certification. For high-care production facilities, travel shall be from high to low care for materials, products, people and waste.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.1.6 Food Processing and Storage areas will be designed to minimize risk of potential contamination and comply with all relevant legislation. - Walls, ceilings, floors, Plastic strip curtains shall be maintained in good condition to prevent pest ingress. - Drains are cleanable, impermeable, and in good state of repair and paint. - Equipment spacing allows cleaning and inspection. - Adequate ventilation controlling air flow, pressure, temperature, humidity, odors and other factors which may impact the quality and safety of the food processing, handling and storage is risk based in design.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.1.7 All utilities to and within the production and storage areas are designed, monitored, and maintained to effectively control the risk of product contamination and employee safety. This includes power, lighting, gases and water.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
6.2 Pest Control
-
6.2.1 Written policy and procedure for managing Pest Control exists. If a Contract Pest Control Company is used, there is a written program defining services. A GSF contact person for Pest control is identified. The contractors will supply current licensing, training, insurance (as well as other licenses required locally) documentation. Note: In countries where there are no local regulations, the supplier shall furnish documentation that pest management professional has formal and ongoing training An in-depth, documented pest management survey assessment shall be undertaken at a frequency based on risk, but at least annually, by a pest management control expert to review the pest management measures in place. The assessment / survey shall: - include provide an in-depth inspection of the site, equipment and facilities for pest activity - review the existing pest management measures in place and make any recommendations for change. The survey assessment shall be timed to allow access to equipment for inspection where a risk of stored product insect infestation exists.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.2.2 A list of approved pesticides/rodenticides for use at the facility is maintained. This includes: - Pesticide name - Target pests - Concentration - amount applied - specific area where pesticide was applied - method of application - Rate of application or dosage - Date and time of application - approved methods and areas of application - instructions for their effective use and action to be taken in case of emergencies - All MSDS/SDS and labels will be present at the facility. - Applicator's signature - EPA or other registration number, as applicable Obsolete pesticide documentation is not commingled with current documentation.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.2.3 A map of the Pest Control Devices (electronic flying insect devices, bait stations, pheromone traps, traps, etc.) is present: - Correctly reflects number & location of traps & is correct and - Up to date. - The map is dated and signed by the employee responsible for administering the program. - There is a documented validation (walk the map to ensure it matches the # and location of each devise) at least annually. Map is to be updated anytime construction to change walls, entrances, ceilings, etc. occurs. It may be labeled as temporary. All map criteria as listed above must be met.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.2.4 All Pest Control activities, deviations, and corrective actions are up to date. Records are to include all application of pesticides, rodenticides and herbicides. Pesticides use conforms with regulatory and label requirements. - In the event of infestation, or evidence of pest activity, immediate action shall be taken to identify at-risk products and to minimize the risk of product contamination. Any potentially affected products should be subject to the non-conforming product procedure.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.2.5 Pest activity is documented and trended. The Pest Control Policy is modified when increased activity is noted and corrective actions are present. If pest activity is identified, it shall not present a risk of contamination to products, raw materials or packaging.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.2.6 No flies, rodents, roaches, lizards, birds etc. are found alive or dead in the production or warehouse areas. No spider / cobwebs.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.2.7 Trap placed on the inside on either side of every exterior door, including dock doors. - All devices are located in such a manner as not to contaminate product, packaging materials or equipment. Internal distance between traps located between 7 – 12 meters (22 – 39 ft) apart. - Bait stations are secured and used only the outside and are between 13 – 30 meters (42 – 98 ft) apart. - Electronic flying insect control and monitoring devices are located at least 3M (9.5 ft) from food contact and ingredient/packaging storage areas. - Any missing or duplicate (extra) devices shall be recorded, reviewed and investigated. - Internal bait stations are inspected at least weekly, with external traps being inspected at least monthly.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.2.8 All pest control devices are properly monitored, maintained and labeled for identification. This includes: - Flying insect killing devices with bulbs changed annually - Pheromone traps replaced quarterly - Rodenticides are not missing or decayed - Sticky paper containing units shall be monitored for insect entry into the plant and changed on a regular basis. All units shall be labeled with date of inspection. Toxic rodent baits shall not be used within production or storage areas where open product is present except when treating an active infestation. Where toxic baits are used, these shall be secured. Electrical fly killer units are not located in direct line of sight from windows/doors. They should be located at least 1 meter (1 foot) away from exposed raw materials, packaging or products.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.2.9 The following will be protected from pest entrance: - All window - Doors - Vents - Dock doors - Air locks - Air curtains - Strip curtains - Roof windows (skylights) etc. All vents, windows, dock doors that are kept open shall be screened. Where hollow or suspended ceilings are used, adequate access to the void must be provided to allow inspection and application of controls. Window / door screens are fully intact. Screens shall be intact and opening of a size so as not to allow flying insects to enter.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.2.10 There shall be an outline of methods used to make staff aware of the bait control program. Outline awareness of staff for training in the use of pest control chemicals.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.2.11 Employees shall understand the signs of pest activity & be aware of the need to report any evidence of pest activity to a designated manager.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.2.12 A Pest Sighting Log book is present for communicating pest activity by GSF personnel to the Pest Control person. The record is monitored an used to initiate necessary follow-up actions.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
6.3 Grounds Maintenance
-
6.3.1 Grounds within 100' (30.5 m) of the building will be free of litter, properly maintained, shrubs are a minimum of 18"(0.5 m) from the exterior wall.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.3.2 Drums, pallets, and idle equipment must be properly stored a minimum of 20' (6 M) from the building. Equipment / parts should be off the ground and stored in a manner as to not allow pest harborage. Specific attention should be paid to bone yards (old equipment is stored in these areas) so that weeds & waste are controlled to prevent the harborage & attraction of pests.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.3.3 To prevent contamination from dust, mud etc. all traffic routes under the control of the company are suitably surfaced and maintained. Driveways / car parking are paved, sealed or suitable surfaced.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.3.4 Exterior areas do not have standing water, leaks, spills or other harborage areas that would be of interest to pests. Drainage of rain water is free flowing. Take into consideration if it has rained recently.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
6.4 Waste
-
6.4.1 Waste disposal complies with all legal and regulatory local requirements. Where appropriate, waste is removed by licensed contractors. Records of removal shall be maintained and available for audit.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.4.2 Waste disposal areas are clean, contained, adequate drainage and adequate size. External waste collection containers and compactors should be closed and/or covered. Outside wet and/or dry waste compactors, modules, and dumpsters are installed and maintained to control leakage or to contain such leakage permitting the container to be removed and the area cleaned. Methods shall define proper storage of waste prior to disposal.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.4.3 Transportation of rubbish or inedible materials does not contact or contaminate: - Finished products - Work-in-progress - Packaging materials - Ingredients.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.4.4 Program is in place to achieve traceability of waste, such as the destination. Branded product should be recorded and the destruction documented. Requirements for the disposal of trademarked waste shall be defined within a contract with the contracted provider & include records of destruction or disposal.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.4.5 Waste materials (trash and inedible waste) are placed in well identified containers, kept clean and clear of production. Waste is removed from the processing areas at regular intervals preventing accumulation. Condemned foods are adequately labeled and or stored in a secured area.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.4.6 Waste handling - collection, storing, disposal, labeling: - Waste disposal systems shall insure that contamination risks to products are minimized through control of cross-contamination & control of pests. - Risk assessment shall consider movement & flow of waste, including dedicated trash receptacles for high or low risk areas & shall not move between the two. - High-risk waste should be transferred to other containers at the high-risk point. - Requirements for the disposal of trademarked waste shall be defined within a contract with the contracted provider & include records of destruction or disposal. - The disposal of trademarked waste complies with customer requirements.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
-
6.4.7 The wastewater treatment plant does not have a negative impact on food safety or quality.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeats -
7.0 Procurement
7.1 Purchasing
-
A documented Purchasing program is written. The methods & responsibility for ensuring all agreements relating to customers: - product requirements including: - Applicable laws and regulations in the market where they will be sold - Laws & regulations that govern food may be through electronic means / a list of regulations - product realization - delivery is specified & agreed shall be documented & implemented. Program must include notification to the customer of any changes to: - customer spec - suppliers - raw materials and packaging and be documented, including: - high fat content - pH - usage conditions such as microwaving - other packaging used on the product - use of recyclable or reusable packaging materials which may affect packaging suitability. Procedures must include: - communication - verification that the manufacturer is aware of the changes and, that - product produced after the change reflects those required changes. Program shall include: - notification by supplier when ownership or - changes in the manufacturing location changes - Emergency purchasing procedures. Produce: risk based fresh produce sourcing: - Transportation - Processing - Cold chain management program. Designed to mitigate the risk of: - micro - foreign material (physical, chemical (allergen, pesticide) contamination) Fresh Produce (YUM!) YUM! FSQA Fresh Produce Testing Expectations - Produce must be shipped in refrigerated trailers with temperatures documented - Transportation of any other foods, packaging and/or other materials in these trailers/bulk containers will ensure no contamination risk of the produce transport - Farms must be in compliance with Good Agricultural Practices (GAP certification) or equivalent GAP program as approved by Yum! QA - Produce transferred directly from the field/packing house to the processor in structurally sound, clean trailer/containers - Product is shipped to processors/restaurants in refrigerated trailers with temperatures documented as per ingredient requirements.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
7.1.2 The company shall control all processes that are critical to the product safety, quality and regulatory compliance to ensure that purchased products & services conform to defined requirements. Contracts or formal agreements shall exist with the suppliers of services that clearly define service expectations and ensure that the potential food safety risks associated with the service have been addressed. Specifications for contract services that have an impact on finished product Food Safety, shall be documented, current, provide a description of the service & detail relevant training criteria of contract personnel. Outsourced services have been evaluated for legality and quality. List includes: - Catering - Uniforms - where protective clothing for high-care / high-risk areas is cleaned by a contracted / in-house laundry, this shall be audited by a third party - Temporary agencies - Vending - Copier - Waste Disposal - Laboratory companies - Contracted maintenance services, etc. The frequency of these audits should be based on risk.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
7.1.3 Effective processes shall be in place for communicating customer specific requirements to the suppliers of raw materials, packaging and services as applicable. - YUM!: Notification of Brand or Business Unit QA by telephone within two hours of incident discovery (leaving a message on voicemail or sending a fax without personal contact is not acceptable). - Decision protocol defining when and if a regulatory agency will be notified.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
7.1.4 Product packaging: Shall be appropriate for the intended use. The supplier of the packaging materials shall be made aware of specifics regarding characteristics of the foods which may affect packaging suitability: - high fat content - pH - usage conditions such as microwaving - other packaging used on the product - use of recyclable or reusable packaging materials - Includes traceability for processing aids and non-conforming product - Packaging shall be stored under conditions to prevent contamination and minimize deterioration. - Certificates of conformity or other evidence shall be available for product packaging to confirm that it complies with relevant food safety legislation & is suitable for its intended use.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
7.1.5 The Supplier must define how they will ensure that product produced by the contract manufacturer meets their customer specifications. A verification schedule, with a sampling plan must be defined. Emergency contact details available: - Name - Phone - Email - Site address - Products supplied - Current list and location of outside storage and cross dock facilities being used for finished product. - Beef & poultry: An SOP shall exist that demonstrates verification of the raw material supplier (for beef and poultry) being checked against the ASL. - Fresh produce & animal protein foods the supplier can trace back to its origin (e.g. field/farm) to the first external customer. - Certificates of conformity or other evidence shall be available for primary packaging to confirm it complies with applicable food safety legislation and is suitable for its intended use.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
7.1.6 Purchasing department has copies of: - raw material - packaging specifications, including primary packaging, lidding, labels, outer cartons. - product / service are available All raw materials, including packaging, are from approved suppliers.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
7.2 Supplier Approval
-
7.2.1 An approved supplier program exists. The approved supplier program shall be based on prior performance of a supplier & the risk level (low, medium, high) of the: - raw mats, including processing aids - ingredients - packaging materials (food and non-food contact), including labels - services supplied. Minimum requirements include: - methods for granting approval including, at a minimum: - methods & frequency for monitoring - methods of reviewing approved supplier performance & status as well as specific manufacturing location. The program must be documented and implemented.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
7.2.2 An approved supplier list for all raw materials, packaging and services is present. List must be kept current & specify manufacturing location. External labs must be included as an Approved Supplier in the master list of Approved Suppliers. The list is: - Specific to each production facility - Emergency contact (at minimum two) details available (name, phone and email) - Site address - Products supplied - Current list and location of outside storage and cross dock facilities being used for finished product.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
7.2.3 Raw materials, services, etc. are only purchased through approved suppliers. For emergency purchasing see 11.2.2.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
7.2.4 Purchased goods supplier performance (Raw materials and packaging) shall be monitored and tracked relative to their performance and compliance to: - quality requirements expectations - specification requirements on an ongoing basis. Criteria shall include : - COA results - non-conformances - customer service response (including documentation) The supplier approval & monitoring system shall ensure any risks, from raw mats, including packaging to the: - Safety - Authenticity - Legality - Quality of the final product - Risk assessments shall be documented
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
7.2.5 Approved suppliers are required to have a food safety audit on file from a recognized auditing body (preferably GFSI). The scope shall include: - Product safety - Traceability - HACCP / Food Safety Plan program - Recall - Hold / release & non-conforming materials program - Sanitation - Pest control - Chemical control - GMPs - Preventive maintenance-• Contamination prevention - Supplier approval - Food defense - Security programs - Food Authenticity Plan Where a supplier has been approved by a questionnaire instead of certification or audit, verification of the supplier's traceability system shall be carried out on first approval & then at least every 3 years. May be achieved by a trace test. Where a raw material is received directly form a farm, further verification of the farm's trace system is not mandatory. Where a valid risk-based justification is provided and the supplier is assessed as low risk only, a completed supplier questionnaire may be used for initial approval. As a minimum, the questionnaire shall have a scope that includes: - Product safety - Product security - Food defense - Product authenticity - Traceability - HACCP review - GMP The questionnaire shall have been reviewed and verified by a demonstrably competent person.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
7.2.6 Feedback shall be provided to the suppliers to facilitate continuous quality improvement. Feedback may be in the form of either or a combination of: - Food Safety audit - Supplier audit - Self-audit questionnaire (only for low-risk packaging) - Includes outside storage and cross-dock facilities - Corrective Action plans shall be available for review.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
7.2.7 Criteria for selecting, evaluating, accepting and rejecting potential and monitoring existing ingredients & packaging suppliers is identified and the criteria is followed & where appropriate, any claims of authenticity, records are kept. For non-food contact packaging a letter of guarantee is acceptable in lieu of COAs unless specified by customer. Guidance: Program must include: an evaluation of ingredient suppliers for all ingredients, packaging (both food contact and non-food contact), processing aids used in ingredients and packaging for production of Yum! Brands products. For non-food contact packaging a letter of guarantee is acceptable in lieu of COA's unless specified by customer. This evaluation shall include: - the country of origin for ingredients - identification of allergens processed on the same lines, and/or facility - food safety audits performed at the ingredient supplier’s facility containing the minimum elements expected in a food safety audit that are listed below: - the Quality Assurance contact - program for the control of non-conforming ingredients and products - vendor risk assessment of their suppliers - preventative maintenance programs - recall program. YUM! FSA: - HACCP - Identification of allergens processed on the same lines, and/or facility - Food Fraud & Food Defense program review including vulnerability assessment for items provided - Supplier food safety culture (e.g. programs, methods and responsibilities related to Food Safety Culture) - Effective traceability system established - Foreign material mitigation program - Visibility of the supply routes - Supplier / 3rd party food safety audit report (for the producing plant). Supplier approval status and risk classification will be assessed: - For high-risk suppliers, minimum annually, low risk supplier frequency defined by the facility - A Farm / Inbound Material Program is established for all Yum produce and includes: 12.1.2 Produce transferred directly from the field / packing house to the processor is structurally sound, clean trailer / containers - Product is shipped to processors / restaurants in refrigerated trailers with temperatures documented as per ingredient requirements.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
7.2.8 Agents and brokers, including wholesalers, shall provide appropriate supplier information and alert sites when deviating from approved suppliers. The identity of the last manufacturer or packer or, for bulk commodity products, the consolidation place of the raw material. Information may be obtained through the agent/broker or directly from the manufacturer, unless the agent / broker is themselves certificated to a GFSI Standard.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
7.2.9 The brand owner is to be notified when any part of the process or packing has been outsourced. The company shall ensure that subcontractors/co-packers are approved & monitored by successful completion to the GFSI. In lieu of a GFSI audit report, the company shall conduct a documented audit of the site. A documented site audit with a scope to mimic that of GFSI shall be completed by a qualified company auditor. The company shall establish inspection & test procedures for products where part of the processing or packing has been outsourced. The brand owner is to be notified when any part of the process or packing has been outsourced. The company shall ensure that subcontractors/co-packers are approved & monitored by successful completion to the GFSI. In lieu of a GFSI audit report, the company shall conduct a documented audit of the site. A documented site audit with a scope to mimic that of GFSI shall be completed by a qualified company auditor. The company shall establish inspection & test procedures for products where part of the processing or packing has been outsourced. A written procedure is documented that assigns responsibility and defines methods to ensure the customer is informed of any changes in materials or process. Process includes moving of product’s manufacture to a new line in an existing facility or to a new facility / different company. The procedure includes notification of QA / PD / NPD of the brand that the product is supplied to. The customer shall confirm authorization to move forward.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
7.3 Traceability
-
7.3.1 The traceability program shall include: - identification of all raw materials - process parameters (for the specific lot) - rework - primary packaging materials (product contact) as well as the - customers to whom the lot was distributed or - the method of disposal. A written procedure outlining who is responsible for maintaining the product identification system is present and shall include the methods used to identify the product. Emergency Contacts shall be current and captured in the customers' system(s). There shall be a minimum of two emergency contacts listed in each facility profile.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
7.3.2 This program shall enable traceability of all components used in the manufacture of: - Specific lot - All raw materials - primary packaging - printed packaging - labels - pre-mixes, including Pre-weigh - rework - work in process, etc. Upon receipt at the facility, the ingredient’s lot number(s) shall be documented. Program must trace one up & one down.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
7.3.3 Where internal plant identification systems are used, these must link back to the original lot code in receipt records. For ingredients that may not have a specific lot number, a method for unique identification and tracing shall be developed and implemented. A written procedure outlining who is responsible for maintaining the product identification system is present and shall include the methods used to identify the product. The test of the traceability system shall include a quantity check / mass balance.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
7.3.4 - Bulk use of ingredients shall be required to have a documented time frame of known use. - Procedures shall be in place for handling bulk material traces and unit load traces. - For bulk or any materials that are “topped off” (new product is placed on top of old product in various types of ingredient holding receptacles), documentation shall be maintained identifying breaks between lots. - Such breaks are typically sanitation or the complete emptying of a bin. - Each component shall be clearly identified and coded to enable traceability back to the lot or source and traceability forward to the material containing the component.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
7.3.5 - A policy is present regarding multiple lots on incoming pallets or shipments. - Suppliers must have secondary supplier traceability requirements/expectations. - Incoming lots of raw material should be limited to no more than three lots per load. - Receipts must identify the lots and quantity per lot. For pallets with multiple lots, a placard is required on the pallet identifying the lots and quantity per lot.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
7.3.6 All inner and outer packages have lot codes on them as appropriate to comply with regulatory and customer requirements. Lot changes during processing are recorded. Traceability shall be maintained to enable linkage back to the date of manufacture and location for all finished packages. Demonstration that the system works properly is present (reports).
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
7.3.7 Representative samples from all lots produced shall be kept until the expiration of the material or as specified by the customer or regulatory authority.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
7.3.8 Traceability procedures must account for: - holdover - batch systems - continuous systems - shrink - product on HOLD - product destroyed - samples - partially used materials - materials returned to the supplier - product salvaged or sold through alternate channels - product in transit - shared systems - donated products - materials that are topped off. - Includes one back and one forward trace for all food products, primary packaging, semi processed product, rework, processing aides and non-conforming product. - For fresh produce and animal protein foods the supplier, in addition, can trace back to its origin (e.g. field/farm) to the first external customer(s). - For bulk ingredients such as products stored / received in silos, the supplier shall a minimum - be able to provide all the lots and quantities that were stored in that silo since the last detail clean up (silo was emptied and cleaned) within 2 hours.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
7.3.9 Included in the traceability program are Product Development Pilot Plant product runs.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
8.0 Material Handling
8.1 Receiving
-
8.1.1 Procedures for inspection, approval & disposition of raw ingredients & packaging material are clearly defined, implemented and reviewed. Findings are documented. A list of Approved raw materials, including primary packaging and requirements for acceptance are available. Acceptance of raw materials (including primary packaging) & their release for use shall be based on one or several: - Detail the receiving procedure for all materials and ingredients that includes Truck inspections (sanitation / condition of the carrier) - Outline of the procedure which places materials that require further testing prior to use, "on-hold" until the appropriate QC inspections can be made - Requiring ‘Letters of Guarantee’ for each ingredient / packaging material supplier - Visual inspection on receipt - Certificates of conformance - Verification of compliance to specification.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
8.1.1.1 Verification of incoming items are compared against the specification: - Labels: - Graphics - Ingredient statements - Allergen declarations - Secondary packaging
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
8.1.2 - Specifics of the procedure which places materials that require further testing prior to use, "on-hold" until the appropriate inspection / testing has been made. Must include methods to prevent release prior to verification of compliance to specification. - Requiring ‘Letters of Guarantee’ and Certificates of Analysis for each ingredient / packaging material. Procedure should include comparison to specification when COA is used for compliance to specification prior to approval and release.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
8.1.3 There shall be a documented procedure for the receipt of allergen containing raw materials. The procedure shall include thelabeling / marking or identification of the raw material, the scheme for segregation & storage of raw materials as well as procedures for transport to and from the processing locations in the facility.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
8.1.4 Materials are reviewed either by testing all key attributes or through COAs, for verifying compliance to specification.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
8.1.5 Key attributes from selected raw materials (based on risk) are identified and to be verified through in-house or outside testing a minimum of yearly and the external lab shall be certified in those tests performed. When COA is used to compare key attributes, procedure shall include comparison to specification prior to release & approval. COA must verify that all attributes were tested and that all are in compliance. Defined limits for relevant attributes of the material (quality / food safety of the final product) shall be included. COA's accompanying each load to have test results for each key attribute or by the testing of all key attributes upon receipt of each load.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
8.1.6 The facility shall have a detailed sampling plan and testing, including frequency and testing methods for in-house or outside laboratory. If received product is to be tested for pathogens or other adulterants, there shall be an effective Test and Hold program.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
8.1.7 A written process of Risk Assessment for Raw Materials, including packaging (food and non-food contact), processing aids, etc. The program ranks raw materials by high or low risk level. The company shall undertake a documented risk assessment of each raw material or group of raw materials & include packaging to identify potential risks. The risk assessments shall be reviewed at three-year intervals, at a minimum, when new literature is issued or at a higher frequency if required as specified by customer or regulatory agency. The risk assessment for a raw material shall be updated: - when there is a change in a raw material, the processing of a raw material, or the supplier of a raw material - if a new risk emerges - following a product recall or withdrawal, where a specific raw material has been implicated - at least every 3 years. The program is to consider: - Counterfeiting - Dilution - Mislabeling - Intentional Adulteration) along with associated mitigations or corrective actions. - Substitution or fraud shall be considered. The company must have a written Vulnerability Assessment Critical Control Points (VACCP) process reviewed yearly that identifies vulnerabilities (such as the potential for: - Where personnel are engaged in vulnerability assessments, the individual or team responsible shall understand potential food fraud risks. This shall include knowledge of raw materials used by the site and the principles of vulnerability assessment. Adulteration and Fraud; to be reviewed whenever there is: - a change in raw material or a supplier of raw materials - emergence of a new risk (e.g. known adulteration of an ingredient or developments in scientific information associated with authenticity of the site’s products or raw materials, - following a significant product safety incident (e.g. a product recall) where the authenticity of the site’s products or raw materials is implicated. Vulnerability assessments are to be reviewed yearly. The analysis must address, at a minimum, the following items: - potential food fraud incidents for similar materials - their suppliers' status and past performance to food safety and quality requirements - control measures taken by the suppliers to ensure integrity of food ingredients/raw materials Processes must be in place to access information to the supply chain which may present a risk of adulteration or substitution of raw materials (i.e. fraudulent raw materials). Such information may come from • trade associations • government sources • private resource centers • activities completed for clause Senior Management Commitment
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
8.1.8 A clearly written documented written returned goods program and procedure is in place to prevent contamination and allergen cross-contact to themselves, the facility and other products.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
8.1.9 When or where required by local legislation, appropriate controls shall be in place for raw materials that are sold with a claim status: - GMO (Bioengineered) - Organic - Irradiation - Gluten-free, etc. - Specific Provenance or Origin - Assured status (Global GAP) - Specifically trademarked ingredients Claim status of each batch of raw materials shall be verified (where claims are made). The facility shall maintain: - purchasing records - traceability of raw material usage and - final product packing records to substantiate claims. The site shall undertake documented mass balance tests at a frequency to meet the particular scheme requirements of any scheme they are certificated to, or at least every 6 months in the absence of a scheme-specific requirement, at least one mass balance test every 6 months.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
8.2 Storage
-
8.2.1 Storage areas are organized and allergens & sensitivities identified. There is physical separation of those items. Products with strong odors shall be segregated to avoid odor migration.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
8.2.2 - Damaged bags or drums must be sealed to prevent product spillage and contamination. - Ingredients contaminated through damage must not be used due to possible extraneous, microbiological or allergen contamination. - Ingredient weighing/handling/storage practices and processing order or change over procedures are to be in place. - Spills must be cleaned up to prevent potential for infestation or cross-contamination. - All necessary steps shall be taken to prevent chemical contamination of the product and written procedures for the prevention of any potential contamination or cross contact (i.e. allergens) shall be established and available for review upon request.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
8.2.3 - Procedures are in place that identify and track shelf life of raw materials and release status of finished goods. - An effective stock rotation system shall be in place. - Ingredients/raw materials and finished products are properly rotated (FIFO or FEFO) and no out of code date material is observed. Exceptions for FIFO or the use of different system, i.e. shipping internationally are described in the FIFO procedure. - Automated systems based off supplier assigned pallet tickets or bar coding must be able to distinguish older product that is received out of rotation, so it is used in production first. If oldest date is not shipped first, written Alternate Minimum Standards for shipping out of rotation must be approved by the appropriate Yum! Brands contact in writing prior to shipment.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
-
8.2.4 The facility will have sufficient space so that all operations & storage may be carried out under hygienic conditions. - Storage racks are clean and clear. - Storage must be off the floor – palletized. - Pallets, racks and equipment shall be maintained in good condition to prevent physical damage (free from nails, splinters, etc.). - In some cases products may be stored on slip-sheets (without pallets) based on the type of product and packaging. - Pallets used for food products must be in good condition: clean, no broken boards, no evidence of mold or infestation, no off odors. - Utilities shall be arranged so that there is minimized opportunity for product contamination. - Raw materials (including packaging) and finished product stored separate from chemicals in clean & dry area. - Spaces for cleaning and inspection, away from walls.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
8.2.5 - Bulk storage of liquid ingredients susceptible to microbiological spoilage shall have adequate controls in place to prevent spoilage or contamination (e.g., insulated, temperature controlled and monitored). - Raw materials, rework, WIP and finished product temperatures are monitored throughout the storage process. - SOPs are defined and present. - A procedure for thawing of frozen products for use in manufacture of product, where applicable, shall be contained in a written procedure.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
8.2.6 - Raw materials stored outside are clean and properly stored. They are checked for cleanliness before they enter the facility. - Pallets stored outside are clean and properly stored. They are checked for cleanliness before they enter the facility.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
8.2.7 - Where appropriate, packaging shall be stored away from raw materials & FP. - Where packaging materials that are not in individual containers (e.g., film roll stock, cartons, etc.) and partially used packaging shall be covered (stretch wrapped or net wrapped) to maintain integrity and prevent potential for contamination. - Packaging, including partially used packaging, shall be clearly Identified to maintain traceability. - Obsolete packaging shall be stored in a separate area & systems shall be in place to prevent accidental use. - Includes labels.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
8.2.8 - There shall be a formal process for the allocation of packaging materials to packing lines & control in the packing area which ensures that only the packaging for immediate use is available to the packaging machines. - Process includes before the packaging run, during the packaging run, at predefined intervals and when printed packaging or labels are brought to the line during the production run and after the run has been completed. - Where off-line coding or printing of packaging materials occur, checks shall be in place that only correctly printed material is available at the packing machines. - Processes shall be in place to check label use is reconciled with expected use and the cause of any inconsistencies investigated.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
8.2.9 - Temperatures of coolers and freezers in the facility are monitored at a pre-determined frequency and records are available. (Continuous recording methods are preferred.) - Alarm systems are utilized for coolers where raw materials, rework, WIP and finished products are stored. - Corrective actions are documented for temperatures outside established alarm settings. Records shall include: -logs of storage temperatures monitored twice per day for temperature sensitive ingredients and finished products, if no continuous recording device, or daily with a continuous on-line recorder - logs for dry storage and non-refrigerated materials, sanitation and general conditions.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
8.2.10 Where storage outside the facility is necessary, yet the items are still on the factory grounds, the items are protected from contamination and deterioration.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
8.2.11 Where outside storage units / facilities are utilized, the same criteria apply as that of internal storage. There should be a list of off-site facilities. The outside or off-site factility shall have: - a valid certification to the applicable GFSI-benchmarked standard or will be in the form of either or a combination of Food safety audit: - third party audit (includes outside storage and cross dock facilities) - supplier audits - self-audit questionnaire (only for low-risk packaging suppliers). - a completed contract or terms and conditions. As a minimum, this will include all the requirements that the company purchasing the storage services must meet for its customers. - supplier shall conduct documented on-site verification, at least quarterly, to confirm off-site storage facilities are compliant to all storage and transport requirements. This shall include: - Sanitation - Storage temperatures for temperature sensitive products - Product shipping temperatures for temperature sensitive products - Transportation temperatures for temperature sensitive product - Trailer security This shall have been reviewed and verified by a demonstrably competent person.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
8.2.12 Cross-dock (pass through) items are considered in procedures. Supplier approval status and risk classification will be assessed. For high risk suppliers minimum annually, low risk supplier frequency defined by the facility.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
8.3 Transportation
-
8.3.1 Incoming vessel (trucks, tankers, railcars, pallets, etc.) inspection program: Written procedures are in place to verify that all incoming raw material carrier vessels are enclosed, sealed/locked, including LTL's. The written procedures address actions to take when seals are missing/broken, or do not match documentation, etc. Deliveries shall be on clean, dry, undamaged pallets (or slip-sheets), free from off-odors and wrapped according to customers specifications. Loading docks are secured to prevent unauthorized delivery. Staged vehicles containing food products shall remain secured while on supplier’s premises. If applicable, procedures are in place to ensure the US FSMA Sanitary Transportation of Human and Animal Food Rule requirements are met (for US FDA regulated facilities only).
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
8.3.2 The facility is registered with the US FDA (Bioterrorism #. where appropriate). The company participates in C-TPAT (Customs Trade Partnership Against Terrorism) as specified by customers. The supplier facility will comply with all relevant legislation and regulatory requirements applicable to the facility and to the market of production and use, which may go beyond specific Yum! audit requirements.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
8.3.3 Tankers shall be dedicated to food only, with records of wash available. Regulatory compliance shall be adhered to; i.e. Kosher.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
8.3.4 Trucks are loaded and unloaded in covered bays where it is a requirement (customer & / or GFSI).
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
8.3.5 Trucks shall be verified to be in good condition, dry, clean and free of off-odors before loading. Lights, where present are intact. The interior (box, trailer, etc.) is in a condition to prevent damage to products during transit. The facility has a documented outgoing trailer inspection. Results and records are maintained. are recorded for both accepted and rejected trailers.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
8.3.6 Procedures in place shall assure that products are pre chilled to required temperature prior to loading, and vehicles are pre chilled prior to loading for transport / distribution (where applicable). - Product temperatures at loading are documented, where required. - Product is loaded so that load is secure (does not move during transport). - Inspection logs are maintained. - Additional requirements for bulk tankers: cleaning certificates shall be available, and verification frequencies for equipment sanitation shall be specified. The frequency must take into account the microbiological - Sensitivity of the material transported. - The frequency must take into account the microbiological sensitivity of the material transported. - Outbound carriers are pre-cooled to 35-45 ⁰F/1.66 ⁰C for refrigerated products, 10⁰F / -12.2⁰C for frozen products. Carrier and product temperatures are recorded on the Bill of Lading. - Carrier and product temperatures are recorded on the Bill of Lading.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
8.3.7 Temperature controlled vehicles must carry suitable on-board temperature monitoring devices. The transport is able to maintain product temperature within specifications. Where required, temperature logging devices that are capable of being confirmed are present. The devices shall be verified at defined intervals.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
9.0 Personnel Practices
9.1 Training
-
9.1.1 Prior to commencing work all staff, including agency supplied staff & contractors, are to have had documented training at a level commensurate with their responsibilities. Staff includes: - Permanent - temporary/seasonal - contractors - delivery personnel who are performing work with: - raw material handling - preparation - processing - packing - storage areas. Training to be considered includes: - company policies on GMP's, Quality and Food Safety Culture - The Golden Standard Policy and audits - basic food hygiene for food handlers - training in areas that impact food safety (including allergens) - including cleaning - machine operation - quality inspections - handling - rework - food safety for maintenance activities - food defense
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.1.2 Training shall include: Pre-requisite programs, GMPs, allergens, hazard analysis, food regulatory requirements, maintenance of food safety plan, customer specifications, specific work instructions for adherence to customer specs & maintenance of the Food Safety plan and quality or process (NOT an inclusive list). - Further training shall be specific to the employee’s position, especially if responsible for the production of certain customers' products. The training program shall include: - Job training methods - Defined Key Competencies - Responsible parties for training - A method to track completion of required training with records maintained - Exit criteria for measuring training effectiveness with records maintained - All training requires exit criteria, in addition where a task is critical to food safety there will be suitable assessment and sign off that the individual is competent in the task required. - All training must be completed by a suitable qualified person. Where this is internal training, the trainer must be able to demonstrate that they are suitable qualified to give the training (i.e. qualified in HACCP training) - There shall be a defined plan for what training is required. - For each individual there will be a record that can demonstrate that all training was completed with relevant documentation (certificate, date of training, title or course, contents, test result, attendance log, trainer sign off, duration of training) - Program should include operators or any other job that has an impact on controlling the KPI's. - There will be a planned review to check that all training is effective and individuals training is reviewed on a timely basis - Training material shall be available and referenced in the individual training records.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.1.3 - Allergen specific training annually for all employees and more frequently for employees who handle allergens. - All relevant personnel, including engineers, agency-supplied staff, temporary staff & contractors, shall have received general allergen awareness training & be trained in the site’s allergen-handling procedures. - Food brought into facility by employees is addressed in training. - Staff should be periodically challenged to test their understanding and refresher training undertaken as appropriate. Documentation is present.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.1.4 Employee training is performed and documented for all toxic, hazardous chemicals before the employee handles the item. GSF Corp: This includes office cleaning chemicals, videojet inks, lubricants, non-food grade materials, lab chemicals, etc.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.1.5 Training completion is documented on the Global Manufacturing Training Matrix within Smartsheet.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.1.6 All personnel shall be adequately supervised throughout the working period with particular attention given to identifying the needs of temporary or seasonal workers and contractors. Exit criteria (what it takes to pass the training) results must be documented.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.1.7 - Training skills register shall include supervisor verification that training was effective. - Where equipment settings are critical to the safety or legality of the product, changes to the equipment settings shall only be completed by trained and authorized staff. Where applicable, controls shall be password-protected or otherwise restricted.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.1.8 - A documented training program that identifies job role competencies is present. - The program ensures that appropriate staff obtain specific competencies, that review of the effectiveness of the training is evident and that staff understands the reason for the training. - The company may use a job matrix to specify the competencies required for roles or for individual staff.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.1.9 Records shall be present in written or oral translation where required. This does not require that translation is necessary but does require that it is established that all staff can understand the instructions to carry out their job role.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.1.10 Work instructions are established for all key processes. These instructions shall include all key operating parameters, equipment settings, job descriptions and qualifications for staff performing key process steps. - There shall be documented job descriptions for staff performing key process steps identified. - Each job description shall include: - Job title - Job summary - a short summary of the overall objective of the job - Job qualification - requirements for job entry - Instructions may be in pictorial format, CCPs may be translated into languages spoken by staff involved in those areas. - Training may be internal or external. - Where a task is critical to food safety, there will be suitable assessment and sign off that the individual is competent in the task required. - Established for all key customer specific processes. Work instructions or Standard Operating Procedures shall include all key operating parameters and equipment settings.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.1.11 - SOPS & work instructions must be written for those tasks that are associated with the Food safety plan. - Training must be conducted with appropriate staff. - Training requirements apply to backup personnel. - Risk Assessment training must be conducted for key team members including but not limited to QA, Maintenance and Operations at a minimum of annually. - Procedures for the control of microbial hazards (external / internal hazards associated with raw animal products and processing equipment).
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.1.12 - The training program must identify the means (format) for achieving refresher training requirement. - Appropriate refresher training, coaching, mentoring or on-the-job experience to improve skills & understanding shall be included in the requirements. Unless otherwise stated all training must be completed at hire and annually (within a 12 month period) thereafter. - Records of training must include the name of the trainee, confirmation of attendance, date and duration of the training, title or course contents & the training provider. - The information may be included in the certificate of course attendance, or in the personnel file. - The name of any translator must also be present.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.1.13 Training records are also required to be present for temporary/seasonal staff and contractors.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.1.14 Training has occurred for the HACCP / Food Safety team & staff involved with CCP's as identified in the HACCP plan that are relevant in the areas in which they may work. The training is documented.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.1.15 A list of qualified employees with HACCP / Food Safety Plan-CCP / Process Preventive Controls monitoring and verification responsibilities is present. Training is specific and more than once per year and proficiency is demonstrated.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.1.16 - Training records are present and current for auditors. - The plant's procedure for Internal Audits must define the competency requirements or aspirations for its Internal Audtors - Competency requirements should include education, training, knowledge and skills required. Experience can be used to justify education requirement. Shall be similar to ISO Lead Auditor (not necessarily certified) or ASQ pre-certification training, or other equivalent training/certification. Attend formal HACCP training from an external HACCP course provider and have risk assessment training or FSMA PCQI (Preventive Control Qualified Individual) as required by US FDA Standards. - Signatures / initials should be matched to the audit reports.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.1.17 All employees hired by the company shall undergo background checks for health (where allowed), drug / illegal substances and include criminal background checks in accordance with local laws and regulations.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
9.2 Hygiene
-
9.2.1 A documented Good Manufacturing Practices Program is in effect and includes: - Buildings - Grounds - Equipment - Processes. GMP's shall be clearly communicated to all personnel, including temporary/seasonal workers, contractors, visitors, etc. Compliance is to be checked on a regular basis and may be through daily/weekly/monthly audits (GMP, Housekeeping audits, etc.). Challenge of personnel at regular intervals, (i.e. microbiological hand swabbing, questioning, etc.) shall be evidence of compliance.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.2.2 Visitors and contractors shall follow all appropriate GMPs'.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.2.3 Signage supporting GMP's are posted in the language(s) appropriate to the facility.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.2.4 - A specific statement identifying exceptions (medical alert bracelets, marriage necklaces, etc.) shall be included in the policy / procedure. - No rings on hands are acceptable. - Rings & studs in exposed parts of the body, including tongue ornaments, shall not be permitted and apply equally to raw material handling, product prep areas, processing, packing & storage areas. REMOVED: Only plain wedding bands (rings) may be worn, no stones or deeply cut rings are acceptable.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.2.5 - Long fingernails, polish/varnish, nail art or false nails are not allowed as they may break or flake off. - Visitors who cannot comply will not be allowed in the affected areas if they cannot comply, gloves may be worn to cover fingers. - Watches and similar wearable devices shall not be worn - Excessive perfume or aftershave shall not be worn.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.2.6 - Hairnets, head coverings, beard nets/shrouds shall be worn, as specified within each facility. - Hair and full beard (includes mustache) restraints made of a fine gauge mesh or solid material. - Baseball caps are not acceptable in production, processing and warehouse areas where exposed product is handled
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.2.7 - There is a glove policy for employees that includes procedures for type of gloves used and their control. - Gloves are provided for operators & there is a procedure for their use and change. Where appropriate, gloves shall be suitable for food use, of a disposable type, of a distinctive colour (blue where possible), be intact. - Where gloves are worn in or around food contact points, they must be of a distinctive color (blue is preferable), be intact and not shed loose fibers. - Where arm sleeves are worn, they must be intact and of a distinctive color where there is food contact. - The procedure defines that gloves are intact, clean and sanitary and not shed loose fibers.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.2.8 - Protective clothing includes uniforms, overalls, head coverings, shoes & boots, aprons, & gloves (whether disposable or washable). - A suitable design of uniform shall be provided to employees, visitors & contractors by the company. - Protective clothing is that which protects the product from contamination; however, the risk assessment shall consider potential contamination from foreign bodies (buttons, fibers from clothing, as well as hair) & therefore protective clothing is likely to be required. - The use of task oriented clothing; i.e. shoe coverings, shoe sanitizer stations shall be utilized as defined in the policy/procedure. Carrying any object in an upper pocket or clipped to shirt of a shirt/coat/uniform is not acceptable - Exceptions for wearing items above the waist may be allowed in the local GMP standard and must be included in the risk assessment. - Applies to all personnel in food processing areas. - Laundering of protective clothing shall take place by an approved contracted or in-house laundry using defined criteria to validate the effectiveness of the laundering process. The process must include: - adequate segregation between dirty and cleaned clothes - effective cleaning of the protective clothing - cleaned clothes are supplied protected from contamination until use (e.g. by the use of covers or bags).
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.2.9 - Designated changing areas shall be provided for all personnel, whether staff, visitor or contractor. - These shall be placed to allow direct access to the production, packaging or storage areas without access to any external area. Clothing material that can cause foreign material contamination is restricted. - Where this is not possible, a risk assessment shall be carried out and procedures implemented accordingly (e.g. the provision of cleaning facilities for footwear). - In high-risk facilities, personnel shall enter by a specially designated changing facility and follow specified procedures for donning visually specific clean overalls, headwear and footwear.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.2.10 When operation contains high care area, a specific order to changing in & out of hairnets & handwashing shall be in place. The changing faculties shall indicate: - how to get in and out of dedicated protective clothing, - site provided footwear which shall not be worn outside the facility, - on entry to high care areas handwashing & disinfection shall be provided & used. - For high care areas, a specific door shall be used to keep protective clothing from being contaminated before entry into the high care area. Footwear Control: - There shall be effective control of footwear to prevent the intro of pathogens into high care areas. - Dedicated footwear shall be worn in the high care area with an effective system to segregate areas for wearing high-risk or other footwear, i.e. a barrier or bench system. - May be by controlled changing of footwear before entering the area or use of controlled & managed boot wash facilities. - A program of environmental monitoring shall be established to assess the effectiveness of footwear controls. - By exception the use of boot wash facilities are accepted where these demonstrably provide an effective control of footwear to prevent the introduction of pathogenic material into high-risk areas. • EMP program shall be established to assess the effectiveness of footwear controls.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.2.11 - Protective clothing worn by staff, visitors and contractors shall be changed at an appropriate frequency, based on risk. - For high-risk and high care areas the protective clothing shall be changed at least daily. Or more frequently, if needed. - Protective equipment shall be maintained in an appropriate condition. When aprons are worn to assemble food products they are to be removed and stored in an appropriate manner (hung on hooks inside the room, etc.). Where items of personal protective clothing that are not suitable for laundering are provided (such as chain mail, gloves and aprons), these shall be cleaned and sanitised at a frequency based on risk. - The design and condition of outer garment should be to prevent contamination of the product; as a minimum containing no external pockets above the waist or sewn-on buttons.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.2.12 - Personnel storage areas shall be designed so that employees have direct access to manufacturing, packaging and storage areas without access to external areas. - Outdoor clothing shall be stored separately from work clothes. - Storage facilities of sufficient size to accommodate personal items shall be provided for all personnel who work within the factory environment. - Outdoor clothing and other personal items shall be stored separately from production clothing within the changing facilities. Facilities shall be available to separate clean and dirty production clothing.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.2.13 - Rest rooms / toilets shall be segregated and not open directly onto the production floor. - Rest rooms / toilets shall provide hand washing to provide sufficient water at appropriate temperatures, single use towels, hand soap, appropriate forced air dryers. - Instructions are posted with toilet facilities in the appropriate language so that all employees can read them - Sinks with soap and water at a suitable temperature - Adequate hand-drying facilities - Restrooms are clean, orderly and well lit - Exhaust is vented to the outside
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.2.14 Lunch rooms shall be separate from the production areas and product. Eating areas in the lunch room and outside the facility shall provide suitable areas with appropriate control of waste. There shall be storage provided to employees for food that is brought in that is not in production, packaging or storage areas.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.2.15 Eating, drinking, chewing gum & electronic cigarettes are not allowed in manufacturing, food handling production, laboratories and storage areas. Drinking of water from purpose-made dispensers and/or by using conical cups or spill-proof containers may be allowed, as long as it's confined to a designated area. Suitable disposal vessels must be provided by the company. Tasting of product in food handling areas is not allowed. Chewing, eating or drinking shall not be permitted other than in designated areas. Spitting is not permitted.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.2.16 Smoking, including e-cigarettes, in countries that allow the practice, shall be adequately controlled so that it does not constitute a risk to the product. Designated controlled smoking areas shall be provided in locations so that smoke cannot reach the product. Adequate hand washing systems for smokers shall be in place.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.2.17 - Band-Aids (Plasters) shall be dispensed through an issue procedure. - The procedure shall be audited and verified. - Be metal detectable. - Shall be of a distinctive color (preferable blue). - Gloves or cots (sheaths) shall be worn when an employee wears a band aid (plaster). - A sample of each lot (batch) of plasters shall be verified as metal detectable by testing through a metal detector. - Records shall be maintained of the testing. - Minor cuts shall have a bandage (colored for ease of identification) and must be covered with a nonporous plastic glove - Personal medicines shall be controlled to insure that they do not constitute a risk to product, the company shall have a documented procedure. - For employees who require medicine to be with them & therefore take medicines, written procedures shall be in place to control the use and storage of these; i.e. a defined requirements to notify the company of the defined medical need.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.2.18 Work areas are orderly with tools & supplies properly stored. Included are: - production shop - maintenance shop - warehouse - storage areas - changing rooms - toilets - outside grounds, etc.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.2.19 - Processes & procedures for managing employee illnesses & communicable diseases shall be: - Established - Documented - Communicated within the company appropriately. - Site shall educate employees on the symptoms of infection, disease or condition which would prevent a person working with open food. - Site shall have a procedure which enables notification of ALL workers of any relevant symptoms, infection, disease or condition with which they may have been in contact or suffering from.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.2.20 - Employees are not to move freely from one process area to another without a garment change where the possibility of cross contamination exists. - The movement of personnel, raw materials, packaging, rework and/or waste shall not compromise the safety of products. - The process flow, together with the use of demonstrably effective procedures, shall be in place to minimize the risk of the contamination of raw materials, intermediate/semi-processed products, packaging and finished products.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.2.21 A site map (diagram) is available that indicates areas of the site and how personnel access & travel between the areas. - Examples of routes that are frequently marked include: - incoming materials - employee movement - finished product movement (to storage) - waste route - low risk zones - high risk zones. - This map shall be cross-referenced with the company controls in place such as what clothing (lab coats, uniforms, etc.) may be worn in which places. - Where personnel have access from one production area to another, transit routes shall be marked. - Access to & through production areas shall be as simple as possible. - Transit & access shall be communicated to visitors & contractors, especially through production areas. - Clear signage is acceptable. - Non-staff personnel who access production/storage areas shall have been made aware of the company's requirements to ensure product safety (GMPs, access, etc.).
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.2.22 Handwash facilities shall be provided at access to and at other appropriate points within production areas. All entries to food handling and storage areas are secured or access restricted / gated.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.2.23 Hand wash facilities shall provide, at a minimum: - sufficient water at appropriate temperatures - knee, foot pedals or automated rather than handles - single use towels, - liquid or foam soap, - paper towel dispensers (touch-free) OR - suitably designed and located air driers - signs (in the appropriate language to the site) to prompt handwashing. High risk areas shall be provided with hands-free operation and sanitizer. There is evidence that hand washing facilities are used.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.2.24 Pre-requisite programs (GMP, Sanitation, etc.): Self inspection program exists. Corrective Actions are documented with responsibility, timeline, completion date and verification. Self-audits are conducted monthly, at a minimum. The program is based on the US 21 CFR 117 Subpart B, Codex Alimentarius or other local regulatory requirements. An audit schedule is developed detailing the scope & frequency of the audits. Facility & equipment must be included as well as who will conduct the audit.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.2.25 There shall be a scheduled program of internal audits throughout the year. Internal audits shall be carried out by appropriately trained auditors & auditors shall be independent; i.e. not audit their own work. GSF Corp FSQR: Internal audits should not occur all on the same day, week or month) spread throughout the year. At a minimum, the program shall include at least four different audit dates spread throughout the year. The frequency at which each activity is audited shall be established in relation to the risks associated with the activity and previous audit performance. All activities relevant to food safety, authenticity, legality and quality shall be covered at least once each year. At a minimum, the scope of the internal audit program shall include, but is not limited to the: - HACCP or food safety plan, including the activities that support it, e.g. supplier approval, corrective actions and verification. - Prerequisite program e.g. hygiene, pest control. - Food defense and food fraud prevention plans. - Procedures implemented to achieve the GFSI certification. - Product / food safety and quality culture plans. - Assessment of the site’s conformity with their food safety and quality management systems. Each internal audit within the program shall have a defined scope and consider a specific activity or a section of the HACCP or food safety plan.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
9.2.26 The Golden Standard Program shall be in compliance with requirements: - Training of employees (minimum of 2 hours) with a minimum of 80% on questionnaire - Training of auditors with proficiency verification annually - Golden Standard cards ae given to employees at completion - Short form audits performed once per shift - Long form audits performed weekly by facility leadership, entire facility must be done monthly - QS team member must perform an audit at least once per shift, a different area of the facility is to be covered - Golden Standard banner shall be signed by personnel and posted in highly visible location (minimum of plant entrance, QS lab, employee breakroom, processing area and warehouse area) - Golden Standard brochures at entrance or reception areas - The Golden Standard Manual shall be available, reviewed and updated based on facility specific requirements - The Golden Standard Manual and training presentation
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
10.0 Equipment Maintenance
10.1 Preventive Maintenance
-
10.1.1 The facility shall have a documented preventive maintenance program. The program includes all equipment and facilities. - The program is managed either manually or computerized. - A master preventive maintenance schedule is maintained including frequencies and status. - There is a plan for internal audit of the preventive maintenance program to ensure compliance to and continual effectiveness of this program. - Includes documented inspections that the factory environment & processing equipment are maintained in a suitable condition: - Hygiene inspections to assess cleaning and housekeeping performance - Fabrication inspections (e.g. doors, walls, facilities and equipment) to identify risks to the product from the building or equipment. The facility maintenance plan will be effectively implemented and routinely verified including maintenance for food safety.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
10.1.2 - A Work Order Policy / Procedure is in place. Work orders are promptly processed and closed. - Work instructions are established for all key processes. These instructions shall include all key operating parameters and equipment settings. - There is a process in place to track overdue / open work orders with a reconciliation program to ensure completion of tasks.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
10.1.3 - Records are maintained for maintenance activities performed, i.e. predictive, preventive, routine, corrective, emergency etc. - Temporary repairs are documented and controlled to ensure that the safety or legality of products is not jeopardized. - These temporary repairs shall be made with approved materials, permanently repaired as soon as practicable and within defined timescale.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
10.1.4 - Qualification and core skills are specified for personnel responsible for performing equipment maintenance. - Training programs are established, and records kept. - Air handling, air exchange, air treatment and ventilation systems shall be accessed by competent personnel only, as part of the facility's planned maintenance program, for adequacy to reduce the potential spread of disease and lower the risk of exposure to infectious microorganisms and allergens.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
10.1.5 - There is a process to track effectiveness of maintenance performed and this information is input for program improvement. Process may include cost, percent of task completion, etc. - Materials and parts used for equipment and plant maintenance shall be of an appropriate grade or quality.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
10.1.6 - A procedure to reconcile all tools after repairs and before start-up is implemented. - Procedure to reconcile all machine parts after repairs and before start-up is implemented. - Post-equipment maintenance inspections are documented. - The program evaluates tools and maintenance workers as potential sources of contamination (physical, chemical, micro) with controls implemented.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
10.1.7 - Maintenance activities in high-risk & high-care areas shall respect the segregation requirements. - Where physical barriers are not in place during the maintenance activities, a documented risk assessment of the potential for cross contamination and an effective, validated process shall be in place to protect equipment from contamination. - Wherever possible, tools & equipment shall be dedicated & be retained in the area.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
10.1.8 Maintenance parts & tools are properly stored (either collectively or by individual maintenance personnel) and responsibility defined for such storage control. - Engineering workshops shall be kept clean and tidy, and controls shall be in place to prevent transfer of engineering debris to production or storage areas. - Training must include process used between QA / Operations / Maintenance or as defined by supplier to sign off that equipment is ready to re-start after maintenance.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
10.2 Equipment
-
10.2.1 - Food processing & packing equipment is well maintained and designed to allow thorough cleaning without dead-ends or dead spots, back-siphonage & other areas that could conceal food and debris. - Equipment, floors, walls and ceilings cleaned as appropriate during production to maintain a hygienic environment and in good condition/repair (e.g. cracks, leaks, unsanitary welds, loose caulking, unsealed control boxes / panels etc.) - Materials and parts used for equipment and plant maintenance shall be of an appropriate grade or quality. Where portable equipment (e.g. handheld devices) and battery charging equipment is used in high-risk or high-care areas, these items shall either be: - visually distinctive and dedicated for use in that area - have specific procedures (e.g. a full clean) to ensure that their use does not result in contamination.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
10.2.1.1 Clean, Inspect and Lubrication (CIL) procedures are in place and documented. CIL should be done at a frequency to prevent defects with a Risk Assessment performed to justify the frequency.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
10.2.1.2 Check weighers within the facility are maintained and checked for functionality. If Check weighers are not performing, then product cannot be produced on the line until repairs are made or an action plan is in place for checks to be in place to cover the check weigher's task. The action plan must be documented with results of checks.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
10.2.1.3 Air knives within the facility are maintained and checked for functionality. If air knives are not performing, then corrective actions must be taken and documented.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
10.2.2 Non-conforming equipment must be identified and placed out of production (may be tagged). Non-conforming equipment includes equipment that, if used, would endanger the safety or regulatory compliance of the product. Records of the disposition of non-conforming product & equipment shall be kept.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
10.2.3 Sanitary Design: The design of new equipment is approved following sanitary considerations. The facility has specific policies & procedures for the review & approval of equipment design before purchase. The procedures utilize and/or reference industry standards such as NSF, 3A, AMA, FDA-SAFE, etc. - Records for purchase of new and/or used equipment verifies that the policy/procedures were followed. - Documented purchase specifications for new equipment detailing site requirements for the equipment. Relevant legislation, food contact surfaces meet legal requirements and details of intended use of the equipment and the type of materials it will be handling. - The supplier must supply evidence prior to supply that the equipment meets customers' requirements prior to supply. - The use of correct parts, seals, etc., material grade (306 / 316 stainless steel), smooth welds / seals where they are exposed to product and could result in physical, chemical or microbiological contamination of the product. Anytime new equipment is installed, replaced or maintained, is there a review for sanitary design. The review can be verified through a review of a Capital project plan or by evaluating new equipment installation or replacement during the facility walkthrough.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
10.2.4 The facility has specific policies / procedures to quality (verify) the sanitary design & installation of new purchased equipment before it is placed into service. A documented, risk-based, commissioning procedure shall be in place to ensure that food safety and integrity is maintained during the installation of new equipment. - Installation work shall be followed by a documented hygiene clearance procedure. - New equipment shall be inspected by an authorized member of staff, before being accepted into operation. - Equipment is put through a test run to determine whether it is cleanable. If the equipment does not meet operating criteria, procedures require corrective actions prior to final approval of newly installed equipment. - The commissioning procedure shall include the update of any other site procedures that are affected by the new equipment, for example, training, operating procedures, cleaning, environmental monitoring, maintenance requirements, schedules or internal audits. - The design and placement of equipment shall ensure that it can be effectively cleaned and maintained. - Mobile equipment is included in the program. - Records are present and accurate.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
10.2.5 Modifications to new & existing equipment do not compromise sanitary design when changes are made to existing equipment, obsolete items are removed (all remnants). - Equipment is designed for effective sanitation and sanitation impact is considered when any upgrades or changes are made to equipment - Change in processing conditions, process flow or equipment shall have had a documented Risk Assessment. - A procedure shall be in place to manage the movement of static equipment in production areas. - Where portable equipment (e.g. handheld devices) and battery charging equipment is used in high-risk or high-care areas, these items shall either be: - Visually distinctive and dedicated for use in that area - Have specific procedures (e.g. a full clean) to ensure that their use does not result in contamination.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
10.2.6 - Welds & seams are of food grade quality (i.e. no tack welds, no welding slag and are smooth). - Pitting is not evident. - Equipment is intact and metal is not split, torn or degraded. There is no metal-to-metal contact (from agitators, etc.).
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
10.2.7 - Idle equipment is stored in a clean condition to discourage rodent / pest activity or harborage. - Includes that pipes and equipment are stored a minimum of six inches (15cm) off of the ground and pipe ends sealed. - Manifold openings are sealed when not in use (capped). - Equipment stored in internal production and storage areas shall be kept clean. - Food contact equipment which has been stored but is not in daily use shall be cleaned and where necessary disinfected, prior to use.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
10.2.8 - Adequate lighting for processing, cleaning and inspection of product is present. - Quality check workstations are conveniently located and well lit. - Protection of lighting is provided against breakage in all production, raw material and finished goods areas. - Quality check workstations are conveniently located and well lit. Where glass lighting is required due to high temperatures (i.e. ovens) this is managed as part of the glass and brittle plastics policy.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
10.2.9 Filtration devices shall have a maintenance record indicating: - Date of the last check - Condition of the equipment or filter - Corrective action when filters need repairing or replacing - Individual performing the function Air System / Emissions / Ventilation: The person responsible for inspection and maintenance shall be a qualified technician (ex: certified HVAC Inspector or have credentials to maintain and repair air handling units. Inspection and maintenance of air handling units / systems shall be a part of the PM program. Air Handling, air exchange, air treatment, ventilation systems shall be assessed for protective ventilation practices and an intervention approach. Includes: - Assessing the potential for increased air exchange - Rebalancing of air movement - Improvement of air filtration if necessary - Proper inspection maintenance - Cleaning, sanitation and disinfection of housing, ducts, filters and grates. The air handling inspection and maintenance must include the inspection and changing of filters, inspection for corrosion, inspection for as-designed air flow and air exchanges, and inspection coils, motors, ducts, and grates for cleaning and sanitation. There shall not be excessive buildup of material on air grates. Sensitive areas: There is positive pressure in the parts of the facility that produce "ready to eat", high care or fully cooked meat products.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
10.2.10 Methods (written SOP's) for monitoring, maintenance of filters & screens shall be developed, responsibility defined & implemented. Calibration is performed on Optical Sorter equipment.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
10.3 Calibration
-
10.3.1 A documented calibration program shall be present. - Procedures are established for the calibration and accuracy of "key" measurement equipment and testing equipment for labs, QC and manufacturing process, including schedules and reference standards. - Program identifies all food safety critical equipment and defines equipment that must be calibrated and functioning for production. - All measuring equipment in the facility is calibrated against a National or International standard and documented. - The uncertainty of calibration shall be considered when equipment is used to assess critical limits. Where no such standards exist, the method of establishing and maintaining the standard for calibration shall be documented. - Documenting methods and frequency of calibration and appropriate reference standard - Calibration frequency is present in the procedures and based on a written risk assessment - Documented list of equipment and its location is present - Identification code and calibration due date - Prevention from adjustment by unauthorized staff - Protection from damage, deterioration or misuse.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
10.3.2 - Process monitoring, such as temperature, time, pressure and chemical properties, shall be implemented, adequately controlled and recorded to ensure that product is produced within the required process specification. - Where monitoring is done, calibration of appropriate equipment is verified. - Measuring and monitoring equipment calibration procedures shall be documented and available to appropriate personnel. Monitoring and measuring devices shall be used and controlled to assure that measurement capability is consistent with the measurement requirements. - The facility shall establish and maintain a master list of all measuring and monitoring equipment that can affect food safety and/or product quality, with appropriate procedures for control and calibration.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
10.3.3 - Third Party calibration frequencies shall be identified for each piece of equipment on the list. - When the measurement equipment is used to measure a CCP, it shall be calibrated at a minimum of once a year. - Each piece of equipment shall have the calibration proof adhered to it. - Results shall be documented. Equipment shall be readable and be of a suitable accuracy for the measurements it is required to perform.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
10.3.4 Calibration shall be performed under suitable environmental conditions, based on stability, purpose and degree of usage of such equipment. Procedures shall define the minimum required accuracy and allowable tolerance of the monitoring and measuring device, outside of which recalibration, repair or replacement is necessary. A calibration / verification program is documented and implemented for freezer & refrigerated storage thermometers, probes, and/or chart recorders.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
10.3.5 Critical measurement equipment must be calibrated at or near the process parameter. Complete documentation of checks shall be maintained including date, initials and actual comparison results, and calibration results indicating the degree of inaccuracy and any adjustments made to bring the equipment back into calibration. Corrective action shall be recorded when the result is out of the tolerance levels. Complete documentation of checks shall be maintained including date, initials and actual comparison results, and calibration results indicating the degree of inaccuracy and any adjustments made to bring the equipment back into calibration.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
10.3.6 Product that may have been affected due to equipment being out of calibration shall be evaluated. If the equipment is used to monitor or measure a CCP, an assessment shall be carried out to determine any potential food safety risk with regard to product tested during the period when the equipment was possibly out of calibration.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
11.0 Food Defense and Security
11.1 Food Defense and Site Security
-
11.1.1 A Food Defense (security) program is written, has been fully implemented at the facility and it meets applicable regulatory requirements (e.g. The US FDA Intentional Adulteration Rule 21CFR Part 121 for US FDA regulated facilities). Note: Threat Assessment has been replaced with ‘Food Defense'. - The facility should have a plan to perform an intrusion test at a frequency consistent with its Threat Assessment. - The facility must have evidence of a drill, simulation or intrusion test to demonstrate that the system has been challenged. The test must take into consideration threat, vulnerabilities and mitigations identified in the Food Defense Plan. The test must be reviewed for effectiveness after it is conducted. The written facility security and food defense plan shall contain a Threat Assessment Critical Control Points (TACCP) process that identifies vulnerabilities along with associated mitigations or corrective actions.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
11.1.2 Food security risk assessment and vulnerability study is completed annually, and corrective actions documented. The Food Defense plan is periodically assessed, reviewed and updated. - The facility security and food defense plans must be reviewed annually or more frequently based upon risk (such as increases in security incidents, and social, political and economic changes). - There must be evidence that at least an annual review is scheduled. This can be verified either by looking at the document review history or by evaluating minutes from the last 3 review meetings. Guidance: A scheduled paper and/or real physical simulation exercise for identified (high-risk to business) possible crisis scenario to be conducted annually. It is important to test all elements of the plan to ensure crisis preparedness. For example, all contacts on contact lists shall be called to assess response time and confirm their role in the plan. External resources such as subject matter experts, legal counsel and public relations firms shall be called to assess responsiveness, availability and their role in the plan. The maintenance of the documentation and results of the simulation exercises are part of the crisis management program. These shall include packaging components, product disposition plans, root-cause analysis and corrective and preventive actions. After the simulation is conducted, there must be evidence of a review / debrief of the process. The site shall determine, what went right, what went wrong and what should be actioned to correct the potential for future failures or hurdles.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
11.1.3 - Industry and government guidelines were used to develop the plan. - In countries, states or territories where there is a legal requirement to premises as a food production site, there shall be documentary proof of the registration. For example, the US Treasury under the 'Public Health Security and Bioterrorism Preparedness and response act of 2002'. - Where applicable, the food Defense plan shall meet the legal requirements in the country of sale or intended use.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
11.1.4 - The titles / positions of individuals who are accountable for coordinating Food (or Product) defense at the facility are listed. - They shall understand potential food Defense risks at the site. This shall include knowledge of both the site and the principles of food defense. Where there is a legal requirement for specific training, this shall be in place. - The individual or team responsible shall understand potential food defense risks at the site. This shall include knowledge of both the site and the principles of food defense. Where there is a legal requirement for specific training, this shall be in place. - Staff shall be trained in site security procedures with annual refresher training.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
11.1.5 The procedures that restrict contractors and visitors access to the facility, in general, including warehouses and/or to individual departments inside the facility / warehouse are defined. - The procedure requires them to report first to a company representative. - A visitor recording system shall be in place. - Access to facility and practices related to security are controlled (employees, visitors, contractors, 3rd party drivers, loss of identification badges, termination of employees, etc.). Contractors and visitors, including drivers, shall be made aware of the procedures for access to the site. - Only authorized personnel have access to production and storage areas. - Contractors working in product processing or storage areas shall be the responsibility of a nominated person. - Incoming ingredients, packaging, and finished product are received in enclosed, secured and/or sealed vehicles / containers / railcars. - In case of seals, the numbers are recorded. Emergency exits are not blocked and are easily accessible at all times. Consider: facility perimeter, storage silos, storage tanks, railcars, ambient and refrigerated trailers, shipping / receiving, raw and finished material storage, production areas, water supply, gas and electric utilities, chemical and hazardous materials storage, laboratories and testing facilities, network servers, proprietary information storage, mailrooms, CCTV monitoring and communication rooms.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
11.1.6 The procedures that restrict employee access to the facility, in general, including warehouses and/or to individual departments inside the facility / warehouse are defined and communicated. - Only authorized personnel have access to production and storage areas. - All entries to food handling and storage areas are secured or access restricted / gated. - Policies and systems shall be in place to ensure that access to the site by employees, contractors and visitors is controlled. A visitor recording system shall be in place. - Contractors and visitors, including drivers, shall be made aware of the procedures for access to the site.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
11.1.7 Staff shall be aware of the company security procedures and be encouraged to make enquiries when unknown persons are observed on site. - "Awareness" includes both a process for new hires as well as ongoing awareness for employees.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
11.1.8 The storage of raw materials, packaging, chemicals and equipment is assessed that procedures are in place to maintain security and prevent potential tampering by unauthorized personnel.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
11.1.9 Where raw materials are identified as being at particular risk of adulteration or substitution (melamine in milk) appropriate assurance and/or testing processes shall be in place to reduce the risk. A documented vulnerability assessment shall be carried out on all food raw materials or groups of raw materials to assess the potential risk of adulteration or substitution. This shall include: - historical evidence of substitution or adulteration - economic factors which may make adulteration or substitution more attractive - ease of access to raw materials through the supply chain - sophistication of routine testing to identify adulterants - the nature of the raw material. The output from this assessment shall be a documented vulnerability assessment plan. This plan shall be kept under review to reflect changing economic circumstances and market intelligence which may alter the potential risks. It shall be formally reviewed annually and whenever there is: - a change in raw material or a supplier of raw materials - emergence of a new risk (e.g. known adulteration of an ingredient or developments in scientific information associated with authenticity of the site’s products or raw materials, for example, information obtained as part of clause 1.1.8) - following a significant product safety incident (e.g. a product recall) where the authenticity of the site’s products or raw materials is implicated.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
11.1.10 Where raw materials or products are identified as being at particular risk, the threat assessment food defense plan shall include controls to mitigate these risks. Where prevention is not sufficient or possible, systems shall be in place to identify any tampering. - These controls shall be monitored, the results documented, and the controls reviewed at least annually. - Incoming raw materials arrive in locked, secure vehicles (company / driver may be bonded). - Partial pallets, single boxes, bins, drums, etc. shall be tamper evident by the use of stretch wrap, specific tamper evident tape and sealed. - Upon receipt, all vehicles / containers / railcars containing incoming ingredients, packaging, and finished product shall be secured with seals and the numbers shall be recorded (seals where required). - Outbound trailers are locked or sealed with seal number recorded upon leaving loading dock. For transportation to company’s own secured storage site, a lock must be used to secure the load. Partial pallets, single boxes, bins, drums, etc. shall be tamper evident by the use of stretch wrap, specific tamper evident tape and sealed.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
11.1.11 - Bulk Raw Materials shall have secured transfer points (i.e. manifolds). - External storage tanks, silos and any intake pipes with an external opening shall be locked. - Loading / unloading of bulk materials shall be supervised. - Dairy transferred in structurally sound, clean, sanitized bulk tank containers (e.g. milk) - Microbiological and analytical testing for dairy products is conducted as required (e.g. regulatory, QAP, specification, etc.) - Produce: Trailers and transport containers maintain required temperature controls - Fresh Produce Testing Expectations: 14.1.3 Procedures are established for the control of microbial hazards (external and internal hazards associated with the raw animal products and processing equipment)
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
11.1.12 - Product authenticity, claims & chain of custody: Systems shall be in place to minimize the risk of purchasing fraudulent or adulterated raw materials & ensure that all product descriptions & claims are legal, accurate & verified. - The site must have a written Vulnerability Assessment Critical Control Points (VACCP) process that identifies vulnerabilities (such as the potential for counterfeiting, dilution, mislabeling and intentional adulteration) along with associated mitigations or corrective actions. - The methodology used to develop the VACCP plan must be traceable to recognized science. - The assessment shall be documented and reviewed periodically.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
11.1.13 Finished products in storage or transport shall be secure to ensure they cannot be contaminated accidentally or deliberately. - This may include tamper evident seals and contractual arrangements with carriers. - All outgoing vehicles (including bulk trailers) shall be locked and/or sealed before leaving the supplier’s dock.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
11.1.14 The Food (or Product) Defense plan requires the investigation and reporting of security breaches to the program coordinators. - All reported or suspected breaches of security are investigated, the cause of any breach is identified, and corrective actions implemented and documented. - When any actual or suspected breach of security occurs, appropriate notification will be promptly carried out, including company management, supply chain partners and/or law enforcement. - If an actual or suspected breach of security occurs an investigation shall be promptly conducted. - Root-cause analysis shall be conducted and corrective actions implemented. - - A detailed, documented incident investigation report is prepared and kept for future reference.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
11.2 Crisis Management
-
11.2.1 A Site Crisis Management Plan (may also be referred to as a 'Business Continuity Plan') shall be written and include a vulnerability (threat) study for any catastrophic exposure, hazard, peril or operational activity that could impair or interrupt the business (site) and supply chain. - The plan shall include, but not be limited to fire, explosion, natural disaster, bomb threat, labor strikes, serious injury, and environmental (spills). - Risks are quantitatively measured (likelihood X severity) and prioritized. Alarms and evacuation plans are detailed. - The plan shall include: raw materials, processing lines, storage, freight management, communication management, trade requirements, etc. - The contingency plan risk assessment includes: geopolitical, business environment, brand, ability to import/export, direct supplier make-up, indirect suppliers make up, distribution & storage, and event-driven risks. - The contingency plan covers raw materials, processing lines, storage, freight management, communication management, trade requirements, etc. - The supplier executed contingency plans tests and are the tests and frequencies aligned with the McDonald's Global or Market Strategic Sourcing Lead. YUM!: Supplier emergency contacts have received annual management training of the Crisis Management Plan.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
11.2.2 Mitigation strategies are in place to either prevent/reduce loss to GSF and rapidly return to normal operation. This can include plans for: - Utility back-ups (power, water, phone / internet) - Electronic records are regularly backed up - Alternate / off-site office space - Emergency raw material supply - Temporary labor resources. - The procedures shall define how exceptions to the supplier approval processes in clause 3.5.1.2 are handled (e.g. where raw material suppliers are prescribed by a customer) or where information for effective supplier approval is not available (e.g. bulk agricultural commodity products) and instead product testing is used to verify product quality and safety. - It shall include all critical functions required during the recovery / restoration phase for business survival. Local crisis communications protocol should be integrated/adhere to the Corporate Crisis Communication Guide maintained by Shellie Frey @ Corporate.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
11.2.3 - Communication internally and externally is clearly defined. - Communication to the media and handling phone inquiry is included. A company spokesperson and backups are clearly defined. - Senior Management will determine when a customer requires notification and comply with the customers guidelines.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
11.2.4 - The plan will identify a coordinator, list of internal titles and positions identified with roles and documented training. - Emergency contact list (include information for Crisis Team members) to include customer, local authorities (governmental and regulatory), management, etc. - A checklist of required activities is developed defining the necessary parties to be informed. Guidance: SQMS v5: 4.3.a Contingency plans shall include the aspects detailed in the Appendix of the McDonald's Global Supply Chain Contingency Policy at a minimum and be aligned with McDonald’s Market / Country Supply Chain & Quality System leads on a routine basis. Guidance: The plant must have access to or possess a hard copy of the current McDonald's Global Supply Chain Contingency Policy. 4.3.b Prior to implementing any alternative or contingency product sourcing (whether raw material, packaging or finished product), McDonald’s shall be involved in the review and approval, per market protocols, of these sources. Contingency plan shall be tested as appropriate with the minimum testing frequency based on risk as described in the McDonald's Global Supply Chain Contingency Policy.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
11.2.5 - Mock (Simulation) exercises to assess the adequacy and efficiency of the Crisis Management plan shall be conducted annually, at a minimum. - Mock exercise for each identified possible crisis scenario is conducted at a frequency commensurate with the respective risk level. Documented results of the exercise with root cause analysis, corrective and preventive actions are present. BRCGS v9: 4.1.f Simulation exercises based on different probable scenarios shall be conducted annually to assess the adequacy and efficiency of the plan. Guidance: A scheduled paper and/or real physical simulation exercise for identified (high risk to business) possible crisis scenario to be conducted annually. It is important to test all elements of the plan to ensure crisis preparedness. For example, all contacts on contact lists shall be called to assess response time and confirm their role in the plan. External resources such as subject matter experts, legal counsel and public relations firms shall be called to assess responsiveness, availability and their role in the plan. The maintenance of the documentation and results of the simulation exercises are part of the crisis management program. These shall include packaging components, product disposition plans, root-cause analysis and corrective and preventive actions. After the simulation is conducted, there must be evidence of a review/debrief of the process. The site shall determine, what went right, what went wrong and what should be actioned to correct the potential for future failures or hurdles.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
11.2.6 - A plan for managing the disruption of product and maintenance of assured supply to the customer is available and is included in the Crisis Management Plan. - If required, plan has been reviewed with appropriate customers and this is verified and documented. BRCGS v9: Guidance, 4.3.a: Contingency plans shall include the aspects detailed in the Appendix of the McDonald's Global Supply Chain Contingency Policy at a minimum and be aligned with McDonald’s Market / Country Supply Chain & Quality System leads on a routine basis. Guidance: The plant must have access to or possess a hard copy of the current McDonald's Global Supply Chain Contingency Policy. 4.3.b: Prior to implementing any alternative or contingency product sourcing (whether raw material, packaging or finished product), McDonald’s shall be involved in the review and approval, per market protocols, of these sources. Contingency plan shall be tested as appropriate with the minimum testing frequency based on risk as described in the McDonald's Global Supply Chain Contingency Policy.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
11.2.7 - The contingency plan has been developed, implemented and maintained for core products and includes results of tests and previous contingency incidents. - The facility should have a plan to perform an intrusion test at a frequency consistent with its Threat Assessment. - The facility security and food defense plans must be reviewed annually or more frequently based upon risk (such as increases in security incidents, and social, political and economic changes). - Reports of the intrusion test shall be documented. - Check if Contingency Test Validation Plan has been completed per customer's requirements - Provide Customer Specific Contingency Test Validation Workbook with signatures (Customer and GSF's) showing test conducted and shared with customer.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
11.3 Recall Management
-
11.3.1 A documented Product Recall Program is implemented to manage incidents that impact food safety and legality of products. The program satisfies the following elements: identification and location of suspected product, the reconciliation and disposition of recovered product, notification of the GFSI certification body such as BRC, SQF, notification of Halal, Kosher certification body (if required), notification to the customer, decision protocol defining when and if a regulatory agency will be notified, outline of responsibilities for individuals on the Recall Team, and who is contacted internally at GSF. Included in the procedure should be sources of legal & expert advice. The Recall program shall be reviewed annually and revised necessary when personnel, procedures, processes, after any GSF recall or as any other factors change. - A Health Hazard Risk Evaluation (HHRE) shall be performed for each incident in order to determine if a recall must be conducted. The Health Hazard Risk Evaluation shall include: - Whether any disease or injuries have already occurred from the use of the product. - Whether any existing conditions could contribute to a clinical situation that could expose humans or animals to a health hazard. Any conclusion shall be supported as completely as possible by scientific documentation and/or statements that the conclusion is the opinion of the individual(s) making the health hazard determination. - Assessment of hazard to various segments of the population, e.g., children, elderly etc., who are expected to be exposed to the product being considered, with particular attention paid to the hazard to those individuals who may be at greatest risk. - Assessment of the degree of seriousness of the health hazard to which the populations at risk would be exposed. - Assessment of the likelihood of occurrence of the hazard. - Assessment of the consequences (immediate or long-range) of occurrence of the hazard. - The HHRE must be reviewed and approved by the Global Sr. VP of FSQR for all incidents.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
11.3.2 Current list of GSF recall team members is available. - Each member shall have a current copy of the Recovery / Withdrawal Program and contact list. - The contact list is current in the customer’s web portal, where appropriate, updated annually, and include: - There must be a minimum of three current emergency contacts identified as an ‘Emergency’ contact and designated with a number that indicates who should be notified first, second and third. - Emergency contacts are aware of their responsibilities to the customer. - The following people must be notified immediately within one hour; VP of Communications, Director of Risk Management and VP/CAO - If a serious incident occurs such as an internal discovery, lab test results, receipt of a customer complaint, a regulatory notification related to alleged illness, adverse events, injury, tampering, adulterated/misbranded products, legal or regulatory action, or insurance claims, the BU Sr. Director/Director of FSQ (if applicable), BU President, Corporate VP / President Protein Products and Operations Services and Global Sr. VP of FSQR must be notified immediately (no later than 2 hours after determining that a potential adulterated/misbranded or non-compliant product has left GSF’s control). - Root cause analysis must be conducted and documented corrective actions shall be implemented for all product recovery/recall incidents. Food Safety Plan reassessment shall be conducted within 30 days of a recall.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
11.3.3 Current list of Customer's emergency 24-hour contacts are available. The list includes: - Appropriate customer contacts - Appropriate distribution contacts, including broker / vendors. - All lists should include contacts for 24 hour / 7 day per week accessibility to a minimum of two individuals. If both a Recovery Team and a Contact list exist, they both shall be included in the program.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
11.3.4 - Current list of Suppliers / Customer's emergency raw materials and packaging supplier 24 hour / 7 days a week contacts are available, sometimes referred to as second tier supplier. - The contact list contains phone / pager numbers for afterhours contact. - The supplier contacts shall have had annual management training in the customer’s Crisis Management Program, where applicable.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
11.3.5 Contact information is current for any certification organization requiring notification. - This is a BRCGS / SQF requirement. - Customer specific contacts to be updated annually. - In the event of an actual recall occurred, the current GFSI certification body is to be informed within 3 working days (using the appropriate directory). - The company shall provide sufficient information to enable the certification body to assess any effects of the incident on the ongoing validity of the current certificate (as detailed in the audit protocol, Part III, section 7.1 - BRCGS). As a minimum, this will include corrective action, root cause analysis and a preventive action plan. - YUM! Brands and McDonald’s must also be notified of any other customer recalls within 24 hours (in addition to the recalls related to their products). YUM! Brands QA shall be notified by telephone within 2 hours of incident discovery. Report any media contact to YUM! Brand QA and delay any contact with media or regulatory officials, unless required by law, until consultation with YUM! Crisis Core Team. Scenarios in which the certification body is to be notified include: - a significant food safety incident (including all product recalls and regulatory food safety enforcement actions) - authenticity or legality incident - including a product recall - regulatory food safety non-conformity (e.g. a regulatory enforcement notice) or - food safety related withdrawal.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
11.3.6 Mock Recovery Program / Traceability Test Program is established and tested two times annually for packaging and ingredients. - The Mock recovery must be conducted at least once each during and after normal business hours. - A mock recovery must track either an ingredient or primary packaging to the customer within 2 hours. - Percent (%) Recovery must meet customer requirements. - Includes a mass / balance exercise - A summary record will be maintained containing: - identification of material - date and time of test - overview of reviewed records - calculation summary - accounts for shrink, rework, product on hold, product sold to direct accounts, product destroyed, samples, partially used materials, materials returned to the supplier, product in transit, product salvaged or sold through alternate channels and donated products. - documented review by the Recall Team - list of who should be notified if this was a real recovery - corrective actions for any failure with a provision for retesting within 30 days for FDA facilities and 60 days for all others.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
12.0 Food Product Protection
12.1 Chemical & Physical Contamination Control
-
12.1.1 - Quality of air, water, steam, processing aids (H2O2, Smart Wash) and gases that come into contact with product are filtered and free of odors. - Air that comes in contact with food or food contact surface is free of oil. Air filtration shall be considered based on the nature of the process & product. Supporting documentation, including that for air filters, is present. Air handling, air exchange, air treatment and ventilation systems shall be accessed by competent personnel only, as part of the facility's PM for adequacy to minimizing the potential spread of disease and lower the risk of exposure to infectious microorganism and allergens. These systems should operate properly as per the design criteria based on the current level of space and occupancy at the facility. The facility should assess these air systems with a mindset of promoting protective ventilation practices and intervention approach. Includes assessment of the potential for increased air exchange, rebalancing of air movement, improvement of air filtration if necessary, proper inspection maintenance, cleaning, sanitation and disinfection of housing, ducts, filters and grates. There is positive pressure in the parts of the facility that produce "ready to eat", high care or fully cooked meat products. - Boiler chemicals that come into contact with steam (culinary steam) are approved & safe for food contact. - Water includes all water used in the manufacture, preparation of product, and hand washing (only potable water shall be used). Water should be available when needed. Boiler chemicals that come into contact with steam (culinary steam) are approved & safe for food contact. Where water is stored and handled onsite (e.g. holding tanks) these shall be managed to minimize food safety risks.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.1.2 An up to date (signed and dated) schematic diagram shall be available of the water distribution on site, including holding tanks, water treatment & water recycling. The diagram shall be used as a basis for water sampling & management of water quality. BRCGS v9: 4.5.2 Map should include the water source 2022 1 Jan YUM! FSA 4.2.6 Control of operations (e.g. security of water and air) P. 4.3.7 Water wells, water storage and water handling facilities are secured
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.1.3 Water pressure / backflow preventers verification is part of a written program. - Records of checks are present, reviewed and signed by management. - Devices shall be installed where necessary, especially in CIP systems & where processing water is recycled. - Backflow devices shall be tested at intervals sufficient to prove that they are operating properly - documentation is present. Records of regular testing are present.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.1.4 Walkways, mezzanines, permanent ladders & conveyors adjacent to or above ingredients, products and product lines are shielded. - They are easy to clean and correctly maintained. - Shielding serves to protect product & packaging materials from possible contamination.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.1.5 A chemical approval program shall be documented. The program is to be utilized for all chemicals purchased by or in use in the facility. Includes: - sanitation chemicals - housekeeping chemicals - boiler chemicals - lubricants - maintenance chemicals - laboratory chemicals - inks (video-jet, etc.) - paint. Strongly scented chemicals are to be avoided. A list shall be maintained of all approved chemicals. A review by an identified, qualified individuals shall occur before the chemical is added to the list. Records of the reviews shall be maintained. The program is audited periodically for effectiveness. -MSDS / SDS and labels, if required, shall be present for all chemicals that are approved. -There is a map that identifies all approved chemical storage and work-in-progress locations.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.1.6 Lubricants - There is a Lubricant Policy / Program. It includes direction that only food grade lubricants are used & stored in food contact areas. - Labels shall state that the lubricant is approved for use in a food manufacturing area or color coding is acceptable - Signage is posted to support the program. - Food grade and non-food grade lubricants are to be stored separately from one another (physical separation, never non-food grade storage above food grade (should be stored separately). - Only food grade lubricants are to be used on equipment. - All chemicals used at the facility shall be purchased, labeled, stored and used in compliance with all applicable laws, regulations and label direction.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.1.7 Laboratory chemical control: All media, reagents & chemicals are received, prepared & stored under appropriate secured conditions, not available to the general work force. - They are dated when opened or prepared and used within their shelf lives. - They are maintained under appropriate temperatures. - Portioned containers, such as pH buffer or media filled test tubes, shall have each portion identified & labeled appropriately with 'use by' dates & lot numbers. - MSDS / SDS's & labels are available for both industrial & commercially purchased products.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.1.8 Operations Supplies - Video-jet supplies & paints including ink, solvents: All areas are free from potentially hazardous materials that may contaminate food product or packing material. They should be securely stored to prevent unauthorized use. Users must have documented training and refresher training in the correct usage of the material. Inkjet ink & cleaners may be stored in packaging areas as long as they are contained in a closed, labeled container.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.1.9 - Drainage shall be designed & maintained to ensure that product contamination risks are minimized, including those from laboratories. - Drainage shall flow from high-risk to low-risk areas. - Process wastewater goes directly to drain to minimize contamination risk. - Waste and overflow water from tubs, tanks, and other equipment shall be discharged directly to the floor drainage system or by an alternative method that meets local regulatory requirements. - Sanitary drainage shall not be connected to any other drains within the premises and shall be directed to a septic tank or a sewerage system in accordance with regulations. - Paths, roadways, and loading and unloading areas shall be maintained so as not to present a hazard to the food safety operations of the premises. They shall be adequately drained to prevent the pooling of water. Drains shall be separate from the site drainage system and regularly cleared of debris. Downspouts are to be considered for drainage consideration. - Discharge from defrost and condensate lines shall be controlled and discharged into the drainage system.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.1.10 Office Chemicals shall be securely stored and have MSDS / SDS available. - It is recommended that chemicals must be approved for use in a food manufacturing facility. If they are not, they should be segregated from food processing / manufacturing chemicals in a secured area. - The usage of the chemical not approved for use in a food manufacturing facility shall not enter the food storage, manufacturing, packaging, labs or maintenance area of the facility.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.1.11 Product liners & bags purchased by the company for use in direct contact with ingredients or WIP shall be appropriately colored. - They shall be resistant to tearing to prevent accidental contamination. - They shall be suitable for food contact.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.1.12 There is a written, documented Glass, Brittle Plastic & Ceramics policy / program, including a map. - Lights & other breakable materials are shielded to prevent potential contamination. - Program includes citation of those items that are plastic but not shielded, including process to follow for cleanup, following breakage. - A list exists of all essential glass, brittle plastics & documentation that the list is audited on a regular basis to ensure that accidental breakage is noted. - Ceramics, at a minimum, are to have a written risk assessment. - Forklift & loading lights are noted in the program. - Included, as well, all lights & glass in areas of production, warehousing, including those that are not adjacent to production & storage. - Scanning devices, radios, etc. are to be included in the program. - Computer or video monitors shall have plastic film (touch screens are excepted), office glass that is not tempered, if glass is present, it is handled in an area that is segregated from other products. - Exception to this is a Fire Extinguisher.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.1.13 Foreign Material Control: There is a documented Foreign Material Program, The site shall establish and implement procedures for the operation and testing of the metal detection or X-ray equipment. This shall include, at a minimum: - responsibilities for the testing of equipment - the operating effectiveness and sensitivity of the equipment and any variation to this for particular products - the methods and frequency of checking the detector - recording of the results of checks. - system must be located after the finished product packing point and in line with the main production flow - Appropriate training records shall be present. - Where foreign material is detected or removed by the equipment, the source of any unexpected material shall be investigated. Information on rejected materials shall be used to identify trends and, where possible, instigate preventive action to reduce the occurrence of contamination by the foreign material. - Metal detection, screens, traps, sieves, magnets, filters (incoming, in-line), X-ray, optical sorter, etc. are included in this program (if applicable). - Metal detection tests for the presence of Fe, Non-Fe, stainless steel are included. Inline tests must use the 5 pass method. - Metal detection devices that are inline must have audible alarms. - Metal detection devices must have positive rejection devices. - Metal detector failsafe features must be tested as part of the validation process and validations should be performed after installation of new equipment, maintenance, relocation of metal detection system, new product or any change to equipment parameter. The failsafe must dump product if inline metal detection is used. - X-rays to be verified at the beginning of production, a minimum of every 2 hours and at the end of production. - X-rays will be calibrated at a minimum of annually that is traceable to a valid national or international standard - Magnets are to be inspected and cleaned at a minimum of once per day. Extraneous material is to be evaluated and documentation of inspection, cleaning and evaluation is to be documented. - Magnets are to have an annual pull hold strength test performed. - Screens, filters and sieves used for foreign-body control shall be of a specified mesh size or gauge and designed to provide the maximum practical protection for the product. - Screens, filters and sieves shall be regularly inspected or tested for damage at a documented frequency based on risk. Records shall be maintained of the checks. - Where defective screens, filters or sieves are identified this shall be recorded and the potential for contamination of products investigated and appropriate action taken. - Procedures are implemented to regularly check, calibrate & clean the equipment. Protein: Where applicable, bone elimination program established to control the risk (e.g. inspection tables, etc.) Guidance: BRCGS v9: 4.10.3.1 Metal detection equipment shall be in place unless risk assessment demonstrates that this does not improve food safety. 4.10.3.4 In addition, where metal detectors are incorporated on conveyors, the test piece shall be passed as close as possible to the least sensitive area of the metal detector (usually the center of the metal detector aperture). 4.10.3.5 X-ray equipment testing procedures shall, at a minimum, include: - Use of test pieces incorporating a sphere of suitable material (e.g. a typical contaminant) of a known diameter selected on the basis of risk. The test pieces shall be marked with the size and type of test material contained. - Tests carried out using separate test pieces. - A test to prove that both the detection and rejection mechanisms are working effectively under normal working conditions. - tests of the X-ray detector by passing successive test packs through the unit at typical line operating speed. - Checks of failsafe systems fitted to the detection and rejection systems. In addition, where X-rays are incorporated on conveyors, the test piece shall be passed as close as possible to the least sensitive area of the X-ray (e.g. this may be close to the X-ray source or close to the X-ray detector). Wherever possible, the test piece shall be inserted within a clearly identified sample pack of the food being produced at the time of the test. Where in-line X-ray detectors are used, the test piece shall be placed in the product flow wherever this is possible and the correct timing of the rejection system to remove identified contamination shall be validated. Testing of in-line detectors shall be completed during both line start-up and at the end of the production period.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.1.14 There is a documented Loose Item Control Policy / Procedure present, with actions to address those risks & clearly define responsibility for the program. - The program addresses proper storage for loose items & stock control. - Utensils and tools are properly stored and responsibility for stock control is defined and includes tool reconciliation. - When loose items are used, they must be reconciled at the end of each production shift. - Missing items (e.g., utensils and tools), are recorded and immediate action taken as per policy/procedure. - Maintenance activities undertaken in high-risk and high-care areas shall respect the segregation requirements of the area. Wherever possible, tools and equipment shall be dedicated for use in that area and retained in the same
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.1.15 A list of registered loose items is used in the processing area. The program addresses documentation and the notification of management when there are missing tools, parts, parts or pieces of tools / equipment.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.1.16 There is a policy / procedure for where wood may be used within the facility. - Wood is controlled so as not to pose a risk of contamination. - Wooden brooms / utensils and wooden benches / tables are not allowed within the production environment. - Wooden pallets are allowed in raw material and finished product storage areas - If wood pallets are used within the facility, controls are in place to prevent product contamination. - Wooden pallets shall be free from damage or splinters, free from taint, and wood treatments where used, are only used in accordance with legislation and approved for food use. Guidance: 2022 1 Jan YUM! FSA 7.2.1 Assurance of zero wooden pallets throughout the processing area where exposed product is handled. Wooden pallets are allowed in raw material and finished product storage areas.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.1.17 - No actual or potential product contamination with biological, chemical or physical hazards are observed. - There are no visible signs of condensation, mold, peeling paint, stained or torn ceiling tiles or rust above any open product or product contact area. - All light shields, gauges, etc. in production over open product areas are intact. - Corrective actions and product released from Hold have had proper dispositions made. - Flexible hosing used to transfer ingredients or product is off of the floor. - Utensils used in product assembly areas are to be clean and stored properly.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.1.18 A Drain Backup Policy / procedure is present, all personnel who could be involved are trained in proper methods to follow.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.1.19 - Laboratory waste shall be handled in such a way that it is secure. - Microbiological waste shall be handled in such a way as to conform to relevant local regulations; i.e. autoclaved, color coded containers, etc.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.1.20 Food ingredients & manufacturing chemicals (solvent, ink, etc.), maintenance materials, etc. that are hazardous shall be handled in such a manner so that all relevant local and federal regulations are complied with. This includes: - specialized storage of used containers - disposal by licensed contractors. Eyewash stations are present and are checked on at least an annual schedule. Records of eyewash station verifications are present.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.1.21 Facility has established a procedure / program to prevent foreign material contamination from water / ice when it is used as an ingredient or is in contact with a food ingredient. - Water / Ice that comes into contact with a food ingredient is potable. - If ice is purchased, it is insured that requirement of facility's requirements are met. - Maintenance records of filtration devices are present. - Records of verification of safety of water / ice are present. - A 10-micron water filter (or smaller) to be used at point of use or earlier in the flow when water/ice come into contact with the food or specifically when water/ice is used as an ingredient. - Ice that used is sourced from an approved supplier and included in the site's food safety risk assessment and to be supplied in appropriate containers. - Produce: Processing water temperatures shall ensure the final product temperature for leafy greens, diced or sliced onions / tomatoes / peppers / carrots, other required items, achieves < or = to 5C / 41F or as required by the customer.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.1.22 Water is tested 2 times each year for: - TPC (APC) - Coliforms - E. coli - lead - mercury - off-flavors and/or odors as a minimum or as required by customer. Testing is conducted by the facility. In surface or bore water usage plants (not city or town water) the facility shall also test for Cryptosporidium or Giardia, unless there is a 2-micron or smaller filter used or the product will be heat processed. All water shall be potable. Guidance: 2022 1 Jan YUM! FSA: 8.4 Water micro and analytical testing program requirements are documented and shall assure the following: 8.4.1 Water, ice and steam (that comes into contact with ingredients or finished product) shall be potable and 8.4.2 With adequate protection through filtration (10 micron or < unless to be used at point of use or earlier in the flow or as otherwise approved by Brands / Us) and backflow prevention. 8.4.3 Water tested at minimum annually (must be drawn from inside the plant from different points of use), supplier shall test for: - Heterotrophic Plate Count - Coliform - E. coli (not necessary if none detected on coliform count) - Nitrates / Nitrites (products affected by Nitrates / Nitrites, example: required for poultry) - Heavy metals: lead and mercury - Off-flavors and odors
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.1.23 Facility has established a procedure / program to prevent foreign material contamination from gases, steam or gaseous refrigerants (nitrogen, carbon dioxide, etc.) when it is used as an ingredient or is in contact with a food ingredient. -Certificate of Analysis or Conformance stating Food or Beverage Grade. or -Supplier provides documents showing the last 3 deliveries are >99.5% pure,
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.1.24 A written Spill Control Program is in place with the relevant procedures for the clean-up and disposal of the chemicals and control material& includes obsolete or out-of-date or empty chemical containers. The written program includes what actions to take when there if a spill and what to do if raw materials, WIP, finished product is affected by the spill. - Secondary containment in place for chemicals stored on-site. - Employee training records are evident. - Spills of allergenic materials shall be taken into consideration and specifics detailed in the procedure.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
12.2 Hold / Release Program
-
12.2.1 There is a documented Hold & Release Program. Procedures exist for the: - Clear identification of the non-conforming product - Notification of appropriate personnel - Secure storage to prevent accidental release, segregation - Defined responsibilities for decision-making in the process, including records - Product returned to the site is managed - Disposition of non-conforming raw materials and finished product. These include an explanation of: - Monitoring - Reworking - How the HOLD will be tracked - Product returned to the site, exceptions and the manner in which product is managed. - Disposal, including records of destruction where a product is destroyed for food safety reasons. In the case of a Class 1 (Food Safety Hazard) hold, when disposition has been made, a physical count of the product on hold is to be performed. It is usual to maintain a log of products which are on hold and to undertake periodic physical checks of held stock to ensure that accidental release has not occurred. The summary of products held, and actions taken. Must be reviewed as part of the management review process.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.2.2 The program defines who can place product on 'Hold' who can release product, how / where Hold product is isolated to prevent accidental release. The product may be held: - Manually - Electronically Where the product is segregated, this is described in the procedure (yellow chains, defined location, etc.) Includes defined responsibilities for the decision-making in the process: - Product release shall include a procedure to confirm that product labels comply with the food legislation that applies in the country of manufacture and the country(ies) of use or sale if known - If product is packaged and distributed in bulk or unlabeled, product information shall be made available to inform customers and/or consumers of the requirements for its safe use. Records shall be present. Any pathogen non-conforming micro for finished product for Yum! Products are fully documented and communicated immediately to Yum! with a documented hold policy and corrective action plan.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.2.3 - The program defines raw materials that are tested for specific bacteria / pathogens of concern are handled within the framework of a documented 'Hold / Release' program. - Incoming raw materials, final product micro & analytical results comply with all customer criteria. - Only dairy products produced from pasteurized milk will be used. In any case where unpasteurized dairy is considered, a risk assessment is required, and use shall be approved by the Global Food Safety Council
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.2.4 - A log of 'Held' products, the reason for the 'Hold' and disposition is present. - It is usual to maintain a log of products which are on hold and to undertake periodic physical checks of held stock to ensure that accidental release has not occurred. The summary of products held and actions taken. - The log must be reviewed as part of the management review process. - When the disposition is to destroy the product, records of the destruction are present.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.2.5 There is a written plan to address, with the customer, any product on Hold that is inadvertently released into the customer's system.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.2.6 - Surplus customer branded products shall be disposed of in accordance with customer specific requirements. - Customer brand names shall be removed from packed surplus products under control of the factory before product enters the supply chain unless authorized otherwise by the customer. - By-products & downgraded / surplus products intended for animal feed shall be segregated from waste & protected from contamination during storage. - Management of surplus food & products for animal feed - Effective processes shall be in place to ensure the safety & legality of by-products of the primary processing activity of the site.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
12.2.7 Where a non-conformity places the safety, legality or quality of products at risk this shall be investigated & recorded to minimize or prevent reoccurrence of events that lead to a 'Hold' decision.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
13.0 Regulatory Compliance
13.1 Regulatory Compliance
-
13.1.1 A program shall be established. It includes identification of ingredients & products containing: - Colors & sensitive agents - Sulfites (in concentrations >10 ppm in the finished product) - FD&C Colors - Gluten and MSG - GMO (Bioengineered) - Irradiation & others as defined by local regulatory or customer requirement. - Whether included in the Allergen Program or not, all criteria included in Section 13.1 are to be applicable to Section 3.2. - The information herein may be included as part of the Allergen Program. Label approvals are documented.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
13.1.2 - For Kosher, Halal & other religious programs, as defined by each facility, all contracts, agreements and certifications will be maintained to ensure compliance with religious authorities. - Appropriate Controls shall be established to ensure the integrity of the product claims. - Appropriate certificates are on-site and are current.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
13.1.3 Regulatory activities shall include a statement of the products Identity Preserved (IP) status of all: - ingredients - additives - preservatives - processing - flavorings. Written procedures must be present detailing how raw materials that are key to IP products are separated to prevent commingling with generic raw materials during: - handling - transport - storage - delivery prior to use. Customer requirements regarding IP status must be included in the IP specification & implemented by the supplier. Where products are labelled or claims are made on finished packs which are dependent on the status of a raw material, the status of each batch of the raw material shall be verified. These claims include: - specific provenance or origin - breed / varietal claims - assured status (e.g. Global G.A.P.) - genetically modified organism (GMO) status (Bioengineered) - identity preserved - named specific trademarked ingredients. The facility shall maintain purchasing records, traceability of raw material usage and final product packing records to substantiate claims. The site shall undertake documented mass balance tests at a frequency to meet the particular scheme requirements of any scheme they are certificated to, or at least every 6 months in the absence of a scheme-specific requirement, at least one mass balance test every 6 months.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
13.1.4 The methods & responsibility are written for the ID & processing of Kosher, Halal, organic, non-GMO food, gluten-free & other products requiring the preservation of their IP status shall be documented & implemented. - Relevant licenses must be obtained if required and accessible for review. - Must have appropriate licenses authorizing use of logos, etc.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
13.1.5 All products shall meet legal requirements in the designated country of use & shall include information to allow: - safe handling - display - storage - preparation - use of the product within the food supply chain or by the customer. There shall be a process to verify that ingredient and allergen labeling is correct based on the product recipe & ingredient specs. There shall be effective processes in place to ensure that labeling information is reviewed whenever changes occur to the - raw materials - product formulation / recipe - process - the supplier of raw materials - country of origin of raw materials - legislation. Where cooking instructions are provided to ensure product safety, they shall be fully validated to ensure that, when the product is cooked according to the instructions, a safe, ready-to-eat product is consistently produced in the country where the product is to be sold. Nutritional information provided to customers/printed on labels shall meet applicable regulatory requirements, and test results shall be available if required (by regulations or customers).
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
13.1.6 - Claim: Where a claim is made, the site shall insure the process is fully validated to meet the stated claim & the effectiveness of the process fully validated. - The process flow for the production of products where claims are made shall be documented and potential areas for contamination or loss of identity identified. Appropriate controls shall be established to ensure the integrity of the product claims.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
13.1.7 Third party regulatory compliance letters are present, where required by customer. Guidance: SQMS: v5: 2.3.1.f If any documents are provided to government authorities for non-routine (see Glossary 9.22) and/or for-cause inspections, supplier management shall promptly communicate to McDonald’s Quality Systems and copies shall be made available to the appropriate McDonald’s Quality Systems representatives upon request. If any in-process or finished product samples of any product manufactured at the facility are taken by government officials or agencies during a non- routine and/or for-cause inspections, the following McDonald’s requirements shall be met. - Duplicate samples shall be taken aseptically, sealed and stored securely in such a way as prevent deterioration or contamination. - McDonald's Quality Systems are to be notified of the samples taken and the agency requesting these.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
13.1.8 All GRAS (Generally Recognized as Safe) ingredients are to have the relevant regulatory citation (not just a reference name and number) present (hard copy or electronic) on file, along with supporting documentation.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
13.2 Document Control
-
13.2.1 An effective Document Control System shall be implemented to ensure that only the correct versions of documents, including recording forms, are available and in use. - Procedures shall be in place to manage all documents which are part of the Food Safety and Quality System. - The method for the identification and authorization of documents shall be documented. - All documents (forms, logs, procedures, etc.) shall be controlled, properly authorized and have proper document control numbers, revision dates. - Revision histories, including the reason for the revision, a list of all controlled documents indicating the latest version / revision number shall be available. - Tracking of copies of controlled documents - Handling of customer initiated or approved revisions - Disposal of obsolete materials - Procedures ensuring authorized individuals have current materials and identify that the location of such materials is controlled, as appropriate. This includes subsequent verification that procedures are effective - All copies of documents and specifications shall have confidentiality statements such as, "Confidential", "Controlled Duplication", "Proprietary Information", "Do not Remove from Facility" - Maintain a "master list" of proprietary documents which shall include: - Reference to all customer proprietary documents (specifications, QA programs, specifications, manuals, formulations, etc.) provided to the supplier including version information. - The location of the documents and all copies - The staff responsible for updating documents for local distribution and local system updates - There shall be a secure process for accessing proprietary documents - Where copying or transcribing proprietary information into facility documents is required for the normal management, these documents shall be considered proprietary as per the procedure.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
13.2.2 - Documents shall be clearly legible, unambiguous, in appropriate languages and sufficiently detailed. - They shall be readily accessible to relevant staff at all times. - Where documents are stored in electronic form these documents shall be stored securely and backed up to prevent loss.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
13.2.3 The reason for any changes or amendments to documents critical to product safety, legality, regulatory compliance, or quality systems and procedures shall be recorded.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
13.2.4 A defined system / procedure shall be in place to ensure obsolete documentation is removed, and when applicable replaced with a revised version. The system is verified on some frequency.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
13.3 Records
-
13.3.1 A procedure that includes responsibility and methods for: - Monitoring - Maintaining - Retaining records is documented and implemented. - Consideration is given to: - Legal - Customer requirements - Maintenance of a master list of records. Included in the written procedure: - Record naming and numbering convention - Procedure for food safety and quality records accuracy, legibility and review, or as required by applicable laws and regulations. - Record retention time period designated or as required by local regulation or the customer, whichever is more stringent - A process to ensure confidentiality and effective: - Storage - Protection - Retrieval - Disposition of records. The procedure shall take into account, where it is specified on the label, the possibility that shelf life may be extended by the consumer (e.g. by freezing).
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
13.3.2 Records are complete, legible, signed (or initialed) or dated; showing monitoring activities have been completed.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
13.3.3 Records are retained according to policy in a secure location for a defined period of time. - Consideration must take into account: - Any legal, customer or corporate requirements - The shelf life of the product, taking into account, the possibility that the shelf life may be extended by the customer (e.g. by freezing). - At a minimum, records shall be retained for the shelf life of the product plus 12 months.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
13.3.4 - Inspection records are reviewed before finished goods are released for delivery. - Critical limits are met and corrective actions and proper product disposition made when these limits are exceeded. - Records are signed and dated by the reviewer, who must be a designated, trained associate.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
13.4 Specifications
-
13.4.1 Finished product specifications shall be: - Documented - Current - Approved by the company - Approved by the customer, where required - Accessible to relevant staff. Shall ensure compliance with applicable safety, legislative & customer expectations. The specifications shall include: - Product quality attributes - Service delivery requirements, where required - Labeling and packaging requirements - Regularly reviewed, preferably annually - Review and amendment history / log available.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
13.4.2 The company shall ensure that any customer specific: - Policies - Requirements - Codes of practice - Methods of working - Storage, etc. are understood, implemented and clearly communicated to relevant staff and where appropriate, suppliers of raw materials and packaging.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
13.4.3 - Manufacturing instructions shall comply with formulas / recipes as detailed in agreed customer specifications and shall be implemented. - Routine inspections by trained and competent personnel shall be conducted to ensure standard operation procedures and processes are followed. Records shall be maintained of nonconformance, root cause analysis, preventive and corrective actions and process improvement steps.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
13.4.4 - Accurate, up-to-date specifications shall be available for all finished products. - These may be in the form of a printed or electronic document, or part of an online specification system. - They shall include key data to meet customer and legal requirements and assist the user in the safe usage of the product.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
13.4.5 There shall be a documented procedure for the amendment and approval of specifications for all parts of the process including regular reviews to ensure adequacy and status. Where documents are stored in electronic form these shall also be: - Stored securely (e.g. with authorized access, control of amendments, or password protected) - Backed-up to prevent loss. Guidance: YUM! QSA 2202 Jan 1: 1.2.4 Reviewed at minimum annually or when any of the following occur, but is not limited to: - Change in ingredients and/or recipe - Change in processing conditions, process flow or equipment - Updated or new processing line for Yum! products since last Yum! audit - Change in food contact packaging, storage or distribution conditions - Change in consumer use of finished product - Emergence of a new risk (e.g. known adulteration of an ingredient) - Responding to customer complaint reviews to prevent reoccurrence - Following a recall / withdrawal - New developments within industry including scientific information associated with ingredients, process or product.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
13.4.6 Specifications and/or their contents shall be accessible to the applicable staff.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
14.0 Customer Focus
14.1 Product Design/ Development & Customer Focus
-
14.1.1 - Customer's product specifications shall be agreed upon and signed by supplier and customer in a separate Food Product Specification document. - Appropriate processes and procedures shall be established and implemented to demonstrate that product specifications are met. GSF CORP: When approval by the customer is reached electronically (email, customer portal, etc.), a screenshot or method to retain the correspondence is present. - Master list of current / active staff with access to customer proprietary documents and systems.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
14.1.2 Product design and development procedures shall be written and in place for new products or processes and any changes to: - Product - Packaging - Manufacturing processes to ensure that safe and legal products are produced. Methods & responsibility for designing, developing & converting product concepts to commercial realization shall be conducted by authorized personnel, documented and implemented. Specific procedures required for transition from pilot plant to in-plant implementation of production. All new products and changes to: - Product formulation - Packaging - Methods of processing shall be formally approved by the HACCP / Food Safety Team Leader or trained designee. The company shall have a procedure for artwork approval and sign-off. When the label information is the responsibility of a customer or a nominated second- or third- party, the company shall provide information: - To enable the label to be accurately created - Whenever a change occurs which may affect the label information. Records of product design shall include: - Formulations - Label compliance - Process flows - Shelf-life trials - Approvals. for all new and existing products shall be maintained.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
14.1.3 Product formulation must be validated on production equipment by facility trials and product testing. Suppliers shall have a full understanding of the microbiological, chemical and physical characteristics of the product throughout its shelf life.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
14.1.4 All process or product specification changes are documented and formally controlled and communicated. Guidance: 2022 1 Jan YUM! FSA: 1.2.4 Reviewed at minimum annually or when any of the following occur, but is not limited to: - Change in ingredients and/or recipe. - Change in processing conditions, process flow or equipment. - Updated or new processing line for Yum! products since last Yum! audit. - Change in food contact packaging, storage or distribution conditions. - Change in consumer use of finished product. - Emergence of a new risk (e.g. known adulteration of an ingredient). - Responding to customer complaint reviews to prevent reoccurrence. - Following a recall / withdrawal. - New developments within industry including scientific information associated with ingredients, process or product.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
14.2 Complaint Handling
-
14.2.1 A Customer Complaint Handling Policy / Procedure is present, and it includes the methods and responsibilities for handling and investigating the cause and resolution of complaints from customers and authorities. Should be communicated to all affected levels. Guidance: SQMS v5: 8.1 Suppliers shall have processes and written procedures in place for measuring customer satisfaction (including McDonald’s staff, distribution centers, and restaurants). Results shall be used to improve product quality and service and be made available for review upon request.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
14.2.2 Root cause analysis and action plans are developed from customer complaints based on seriousness of the incident. There shall be analysis of customer complaints and opportunities for improvement identified.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
14.2.3 - Where there has been a significant increase in a complaint or a serious complaint root cause analysis shall be used to implement on going improvements to product safety, legality & quality and to avoid reoccurrence. - The effectiveness of the corrective actions to prevent reoccurrence is documented. - Reporting will be made to relevant staff.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
14.2.4 - Customer complaints are recorded and trended. - The complaints shall be analyzed for significant trends. When there has been a significant increase of a complaint or serious complaint a root cause analysis shall be carried out. - Where illness or foreign material complaints occur, the analysis should be expanded to identify the cause and corrective actions. - Trends are monitored. Guidance: SQMS v5: 8.1.b. Conducting ATCQ: Where applicable, the supplier is actively involved in performing Restaurant Operations Improvement Program (ROIP), Across The Counter Quality (ATCQ), Behind The Counter Quality (BTCQ) and working with McDonald's on improving product quality and service.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
14.3 Change Management
-
14.3.1 Customers are notified when any change is made in the process or profile of the product. Where required the notification is in writing. This includes: - Raw materials - Packaging - Formulation - Processing procedures (sequence of events) or line on which the product will be made, emergency process changes - Regulatory status - When non-conforming product is inadvertently released into the system - When the ability to supply product is temporarily halted - A failed or expired GFSI audit - Equipment changes. Where the label information is the responsibility of a customer or a nominated second- or third- party, the company shall provide information: - To enable the label to be accurately created. - Whenever a change occurs which may affect the label information.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
14.3.2 There shall be a written procedure for changes to: - Processes - Procedures - Structure (construction) that ensures that customer & regulatory compliance are in alignment.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
14.3.3 Changes to processes or procedures are assessed to ensure controls are effective. Changes include those to: - Specifications - Materials - Equipment - Resources. The changes are evaluated for their impact on quality and communicated to customers.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
14.4 Sensory
-
14.4.1 A written Sensory Program is present. The program includes the following: - Product reviews at set intervals - Products are tested against targets or reference samples - Sensory results are documented - Reported and communicated to Sr. Management on a regular basis. Trends are cited & corrective actions implemented. Where online sensory analysis is required, this shall be covered in the policy giving clear direction. Guidance: SQMS v5: 7.4 Sensory Attributes and Evaluations: - Processes shall be in place to understand the product performance at McDonald’s restaurant. - Suppliers shall follow McDonald’s guidelines on sensory evaluation, utilizing McDonald’s Global Scoring Method: Degree of Difference 9-point scale methodology and McDonald’s standard, approved kitchen equipment and procedures to cook and hold products according to McDonald’s Operations and Training Manual. - Processes and procedures shall be established and implemented to demonstrate that McDonald’s requirements on product sensory attributes are met. Guidance: F397 There is a program in place to conduct sensory evaluation for product(s) manufactured aligned with McDonald's Quality Centers and Quality Leads. Supplier shall have a documented process in place to demonstrating product performance at McDonald's restaurants. Product sensory tests are performed by trained persons prior to release of a production run. Additionally full product sensory evaluations of products are performed by trained panelists, on a periodic basis comparing products against target or in-house reference samples. This will be documented and available for review upon request. McDonald's Quality Centers current sensory tools, product specific evaluation forms (scoresheets) and SOPs are used for evaluation. In-plant evaluations are conducted using approved kitchen equipment and procedures to cook and hold products according to McDonald’s Operations and Training Manual. A process is defined for selection of in-house reference / target. Evaluation results are effectively communicated to McDonald's market QS or global QS leads and action taken for continuous quality improvement. For additional guidance on in-plant sensory requirements, please refer to McDonald’s Global Sensory Manual, chapter 5: In-Plant Sensory Evaluation for Quality Control or contact your Quality Lead. SQF v9: 7.4 Sensory Attributes and Evaluations - Specific McDonalds requirements
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
14.4.2 Sensory Evaluation Program: There is a program in place to conduct sensory evaluation per the product specific "critical to product quality attributes" as agreed with the customer. Note: this program is independent from the off-line QC and ‘At Time of Manufacture’ (ATOM) program. - Product sensory evaluations are performed by at least three (3) trained panelists. - The Sensory equipment & area is maintained in a clean condition.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
14.4.3 The most current product specific evaluation forms are used for evaluation.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
14.4.4 Products samples are compared with target or in-house reference samples. A process is defined for selection of in house reference / target. McD's Global Sensory Manual 2022 1 Jan YUM! FSA: 11.3.8 Will have retained samples which can be used for inspection as required by Brand / BU QA
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
14.4.5 Evaluation results are effectively communicated and action taken for continuous quality improvement. Minimum acceptable scoring is set as a goal to meet and/or exceed customer's expectations.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
14.4.6 There is a written shelf-life determination program that defines specific protocols and reflect conditions expected during all stages of the product life. - Specific customer requirements for shelf-life determination must be met. - Shelf-life studies shall be conducted when the product has been developed, then repeated at a pre-determined frequency or when formula changes occur. - Secondary shelf-life studies are conducted for every product or group of 'like' products on a regular basis according to customer requirements. - Shelf-life studies or related data/reporting is no more than five (5) years old or are executed more frequently at the request of the customer. - Records are on file and available which establish the scientific basis for the finished product shelf life. - Chemistry and microbiology of the product undergoing the studies is generated at intervals as specified by internal protocol or the customer. - A report is to be generated at the conclusion of the shelf-life study
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
14.4.7 - Where shelf-life trials are impractical prior to production (for instance for some long-life products) or not required, a documented science-based justification for the assigned shelf-life shall be produced. - Supporting documentation must be present. - Records on file may be used to establish scientific basis for finished product shelf life of similar products.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
14.4.8 - Shelf life shall be based on risk & include sensory analysis and as applicable microbiological testing and relevant chemical factors. - Records & results from shelf-life tests shall verify the shelf-life period indicated on the product. - For McD's products shelf-life studies or related data / reporting is no more than five (5) years old.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
14.4.9 - Sensory panelists are trained and records are present. - Retraining occurs on a regular (minimum of once per year). - Lighting of sensory area meets customer standards. - McD’s: The evaluation room should have adequate lighting for assessing the appearance of the product. Cool, white lights are recommended for evaluation rooms. Recommended lighting is full spectrum, cool daylight at 5000 – 6500 K. Certain products are evaluated with McDonald color guides and require consistent lighting specifications.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
15.0 Food Safety Culture
15.1 Food Safety Culture
-
Assessment Guidance: Evaluate the following items to assess if the facility has a strong food safety culture to execute food safety policies and procedures that govern plant operations.
-
15.1.1 The facility shall establish a food safety culture program. Senior management shall provide evidence of the commitment to implementing, maintaining and enhancing the food safety culture. SQMS v5: 3.2 [Not Scorable in 2022]: A "near miss" reporting system for food safety non-conformance must be in place and linked to a review, action process and communication plan. Guidance: Does the site track food safety "near misses" and are these used as a leading indicator to understand the potential for food safety incidents?
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
15.1.2 Have all food safety non-conformities identified in the previous audits been effectively addressed to prevent reoccurrence? Review the plants training plan and determine if actions and changes identified in the food safety culture improvement plan is included in ongoing training.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
15.1.3 Do employees at all levels have the necessary training and skills to be empowered to promote and execute good food safety decisions and practices? BRCGS v9: 1.2.2 All employees shall have access to relevant documentation these and be able to request an assessment of training needs for activities undertaken to maintain product safety, authenticity, legality and quality.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
15.1.4 Do employees have the freedom to act when any food safety issues are noticed (e.g. stopping a line)?
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
Corrective action
-
Preventive action -
-
Repeat -
-
15.1.5 Are whistleblower procedures established in support of food safety? BRCGS v9: 1.1.6 The company shall have a confidential reporting system to enable staff to report concerns relating to product safety, authenticity, integrity, quality and legality. The mechanism (e.g. the relevant telephone number) for reporting concerns shall be clearly communicated to staff. The company’s senior management shall have a process for assessing any concerns raised. Records of the assessment and, where appropriate, actions taken, shall be documented.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
15.1.6 Is evidence of management commitment to food safety and quality available? - Methods and responsibilities related to Food Safety Culture are defined and shall include: - Policy / program on leadership responsibilities in support of Food Safety Culture is established. - Senior management reviews results (within 30 days) of internal customer specific self-audit and all external food safety audits. - Ensures all identified foot safety non-conformities in the above audits are effectively addressed to prevent recurrence. - Defined tools used to measure the facilities food safety culture (e.g. employee food safety culture surveys, other employee feedback gathering mechanisms) and follow-up actions needed. - If completed by Brand / BU, was progress made to the customer specific Food Safety Culture and People Capability Assessment. - Whistleblower procedures are established in support of food safety. - Reviewed annually by senior management.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
15.1.7 How does facility ensure food safety is never compromised when considering actions including margin improvements, cost reductions? 2022 1 Jan YUM! FSA: Food Safety Culture A positive food safety culture mitigates the risk of a foodborne illness and food safety incidents. Research supports that the most important factors for developing a food safety culture are senior leadership and management commitment and worker food safety behaviors. If leadership / management creates a work environment that encourages proactive food safety behavior and food safety culture, the plant teams will be encouraged to demonstrate proactive food safety behavior. Consistency by senior leaders and management commitment plays a key role in setting the example for the plant employees to follow. Have you ever wondered why some people do the right thing all the time and others only when their supervisors are watching them? Food safety culture is a shared attitude by all employees (leadership, managers and team members), especially with new employees who will tend to follow the dominant behaviors found in the facility.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
15.1.8 Are system failures with potential impact on food safety taken seriously and are used as continuous improvement opportunities?
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
15.1.9 Does facility have processes in place to ensure food safety is built into the innovation process?
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
15.1.10 Does the innovation process / team include a food safety representative?
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
15.2 Food Safety Culture Employee Interviews
-
15.2.1 What does food safety mean for you?
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
15.2.2 Who is responsible for food safety in this facility?
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
15.2.3 Would you eat or feed your family the products made at this location?
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
15.3 Additional Interview Questions
-
15.3 Additional food safety culture questions to ask employees during the assessment (select a minimum of 6 questions):
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
15.3.1 Are you comfortable with reporting a food safety issue to facility leadership? Employees shall be aware of the need to report any evidence of unsafe or out-of-specification product or raw materials, to a designated manager to enable the resolution of issues requiring immediate action.
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
15.3.2 Are responsibilities for food safety clearly defined?
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
15.3.3 Do you know 1 food safety measure / enhancement implemented at this site?
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
15.3.4 Are the relationships between the Quality and Food Safety function and other functional teams adversarial or collaborative?
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
15.3.5 Do new employees receive food safety training before they are allowed to work in the facility?
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
15.3.6 Do you appreciate when a co-worker points out to you if they notice that you are doing something that could affect food safety?
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
15.3.7 Do you believe that your supervisor always puts food safety ahead of production?
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
15.3.8 Are you comfortable stopping the line whenever you see something that might harm the quality and/or safety of the food we make?
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
15.3.9 Do you understand how food can get contaminated with bacteria or allergens that can make people sick?
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
15.3.10 Do you understand how the plant measures the food safety performance and how the facility is doing?
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
15.3.11 Do the facility decisions, actions and behaviors change when the facility is audited?
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
15.3.12 Are the food safety expectations / rules reviewed with you regularly?
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
15.3.13 If you make a suggestion that will improve food safety, do you believe it will be taken seriously by the facility leadership team?
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
15.3.14 Do you believe that food safety is a very high priority for leadership at this plant?
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
15.3.15 Do employees at this facility get recognized for their contribution to making sure that we produce safe food?
- Critical
- Major
- Minor
- Recommendation
- BP
- Compliant
- N / A
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
Corrective action -
-
Preventive action -
-
Repeat -
-
15.4.1 Enter the overall Food Safety Culture evaluation results in the "Comments" section (e.g. the facility has a strong food safety culture)
Sign Off
-
Prepared by: