Title Page
-
If the site is not listed please fill it in.
-
Conducted on
-
Prepared by
Location
-
Building
-
Room numbers
-
Lab representative
-
-
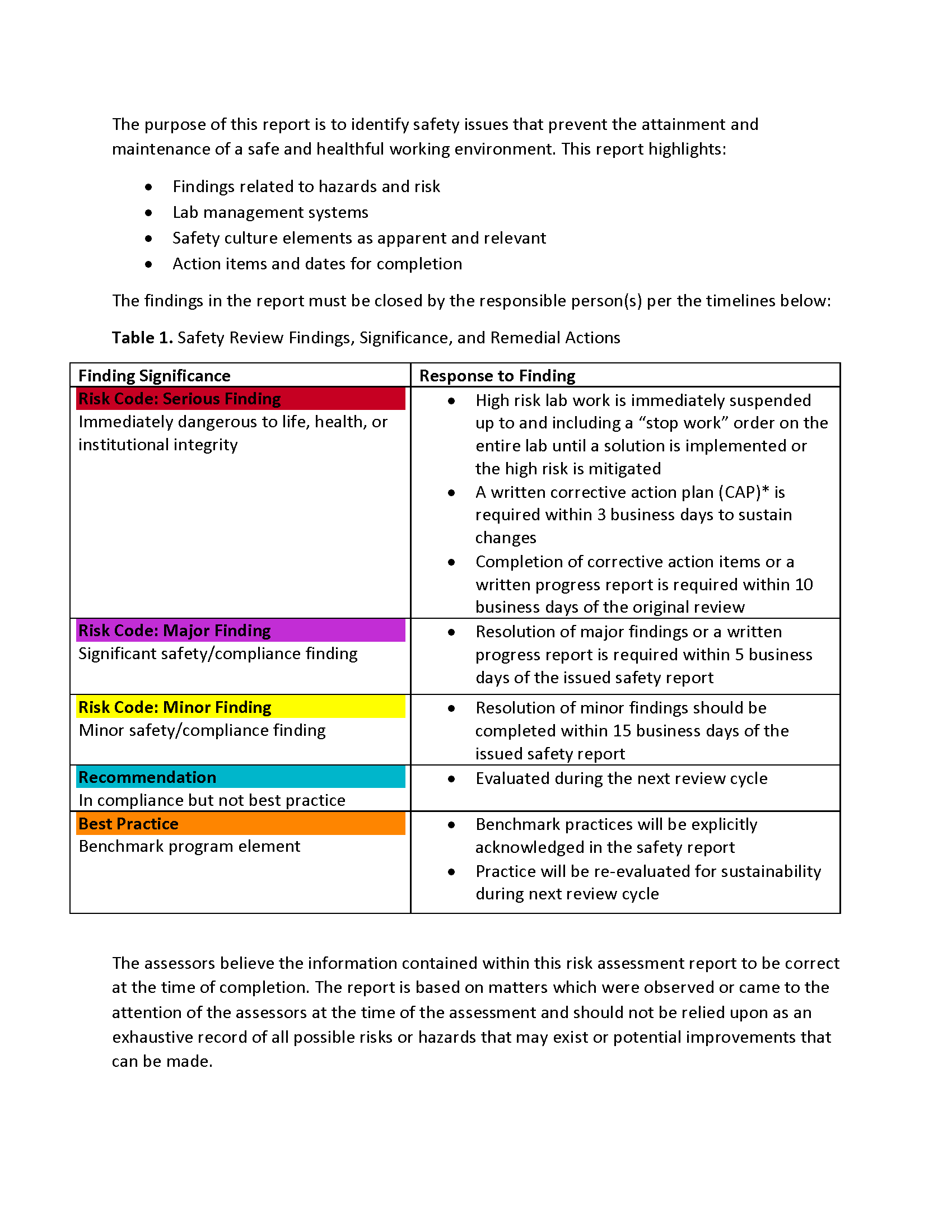
Additional detail on risk coding/corrective action timelines for failed items are in the report below.
Signage
-
Is laboratory door placard present at entrance(s) to the lab?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 45, 13.2.1 Corrective Action: All laboratories and lab support spaces must have caution signs at entrances to advise entrants of the hazards present in the rooms and identify appropriate individuals for routine and emergency contact. Request caution signs online at [https://ehs.utk.edu/index.php/laboratory-safety/lab-safety-administration/lab-door-placards/] or by contacting EHS at (865-974-5084 or ehs_labsafety@utk.edu).
-
Is contact information on laboratory caution sign(s) up-to-date?
-
Risk +
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 45, 13.2.1
Corrective Action: All laboratories and lab support spaces must have up-to-date caution signs at entrances to advise entrants of the hazards present in the rooms and identify appropriate individuals for routine and emergency contact. Update caution signs online at [https://ehs.utk.edu/index.php/laboratory-safety/lab-safety-administration/lab-door-placards/] or by contacting EHS at [865-974-5084] or [ehs_labsafety@utk.edu]. -
Is hazard information on the laboratory caution sign(s) current?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 45, 13.2.1
Corrective Action: All laboratories and lab support spaces must have up-to-date caution signs at entrances to advise entrants of the hazards present in the rooms and identify appropriate individuals for routine and emergency contact. Update caution signs online at [https://ehs.utk.edu/index.php/laboratory-safety/lab-safety-administration/lab-door-placards/] or by contacting EHS at (865-974-5084 or ehs_labsafety@utk.edu).
Biosafety
-
Is this a lab that manipulates biological materials?
-
Which type of lab?
-
Registration Number:
-
Is the current version of the biohazard signage present at entrances to BSL-2 areas?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: CDC/NIH BMBL 6th ed Corrective Action: Ensure the current format of BSL-2 biohazard sign is posted in the appropriate place and contains current contact information. (https://biosafety.utk.edu/biosafety-program/forms/request-a-research-door-sign/)
-
Is the current version of the Infectious Material Exposure Response Procedure posted?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: CDC/NIH BMBL 6th ed
Corrective Action: Ensure that laboratory personnel receives appropriate training regarding hazard/exposure evaluation procedures and necessary precautions to minimize exposures. -
Are biohazard labels prominently displayed where needed?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: CDC/NIH BMBL 6th ed Corrective Action: Label equipment such as refrigerators, incubators, freezers, centrifuges, etc. which are used to store biological materials hazardous to humans or may be contaminated with biohazardous materials. Post biohazard stickers so that they are clearly visible.
-
Are biological waste containers for solid non-sharps waste properly labeled, leakproof, lined with a biohazard bag, equipped with a closure, and used correctly (managed to prevent overfilling, minimal liquids, closed when not in use, etc.)?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: CDC/NIH BMBL 6th ed
Corrective Action: Solid biohazardous waste is collected for final treatment and disposal in a leak-proof container lined with an autoclave bag of moderate thickness to prevent punctures. The collection container must have a lid or other means of closure and the container must be labeled with the biohazard symbol regardless of the containment level of the lab. -
Are items that could puncture the autoclave bag in the biohazardous waste containers (i.e., pipette tips and serological pipettes) secured in such a way as to minimize the puncture potential?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: CDC/NIH BMBL 6th ed
Corrective Action: Pipette tips must be collected in a manner that ensures their containment when placed in the larger biohazardous waste container in the lab. The method for containing the tips is at the discretion of lab personnel as long as the risk of punctures or tears from pipette tips is eliminated. -
Are personnel familiar with the proper decontamination strategies for biological waste (e.g., cultures, stocks) prior to disposal?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: CDC/NIH BMBL 6th ed
Corrective Action: Ensure validated methods for infectious material disinfection of biological materials are used.
Biosafety Cabinets
-
Have biosafety cabinets been certified upon initial installation, after being physically moved or relocated, or within the past 12 months?
- Best Practice
- Yes
- Recommended Improvement
- No
- N/A
-
Date of the last certificaiton:
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: CDC/NIH BMBL 6th ed Corrective Action: Follow guidelines for BSC certification. Contact EHS Office (865-974-5084) for guidance or at (ehs.utk.edu).
-
Is all equipment properly cleaned, disinfected and/or decontaminated?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: CDC/NIH BMBL 5th ed. Corrective Action: Clean and decontaminate equipment regularly.
-
Are disinfectants prepared and used properly?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: CDC/NIH BMBL 6th ed. Corrective Action: Clean work surfaces after completion of work and after any spill or splash of potentially infectious material with appropriate disinfectant using the appropriate contact time.
-
Is a biological spill response plan available and have lab members been trained?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: CDC/NIH BMBL 6th ed. Corrective Action: Establish a lab-specific spill response plan. Train all lab members and post or file in a conspicuous location. (https://biosafety.utk.edu/biosafety-program/emergency-response/biohazardous-spills/)
-
Are aerosol control methods in place to prevent infectious aerosol exposure?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: CDC/NIH BMBL 6th ed. Corrective Action: Conduct infectious aerosol-generating work inside a biosafety cabinet. Ensure centrifuges are equipped with bio seal rotors or safety cups. Ensure other aerosol-generating procedures are conducted in such a way as to reduce the risk of aerosol exposure. Consult EHS (865-974-5084 or ehs_labsafety@utk.edu) for situation-specific questions.
-
Are vacuum lines equipped with liquid waste decontamination traps properly housed in a spill proof container?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: CDC/NIH BMBL 6th ed.
Corrective Action: Ensure vacuum lines used to aspirate biological materials are equipped with a liquid disinfectant trap (in secondary containment, if not contained in a biosafety cabinet). -
Are personnel aware of how to properly store and transport samples (i.e., capped, leak-proof primary container, with leakproof secondary container)?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: CDC/NIH BMBL 6th ed.
Corrective Action: Transport infectious materials and potentially infectious materials in sealed primary containers and sealed, leakproof secondary containers. -
Are needles being bent, broken, sheared, or otherwise manipulated by hand?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: CDC/NIH BMBL 6th ed.
Corrective Action: Eliminate unnecessary manipulation of needles. If necessary, employ engineering controls with research materials to reduce a puncture risk. -
Is there a documented exemption in place when recapping needles used for procedures requiring BSL-2 containment?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: CDC/NIH BMBL 6th ed. Corrective Action: Do not recap needles used for procedures carried out at BSL-2 unless a specific exemption is in place. Contact EHS for assistance with the exemption process (865-974-5084 or ehs_labsafety@utk.edu).
-
If allowed for procedures at BSL-1, is the one-handed scoop technique or a mechanical device being employed for needle recapping?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: CDC/NIH BMBL 6th ed. Corrective Action: At BSL-1, do not recap needles unless a safe sharps handling technique such as the one-handed scoop or mechanical device is being used.
-
Are biologically contaminated needles and other sharps such as broken glass, Pasteur pipettes, microscope slides, etc., disposed of in appropriate sharps containers?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: CDC/NIH BMBL 6th ed. Corrective Action: At a minimum, place biologically contaminated sharps in a properly labeled solid-walled biohazardous sharps container. Ideally, all sharps may be collected in the same manner as biologically contaminated sharps.
-
Are biologically contaminated sharps containers closed and removed for disposal when they are approximately 3/4 or when items do not freely fall into the container?
-
Regulatory Citation: CDC/NIH BMBL 6th ed. <br>Corrective Action: All sharps containers must be permanently closed when 3/4 or when items do not freely fall into the container.
-
Are biohazardous sharps containers disposed of properly?
-
Regulatory Citation: CDC/NIH BMBL 6th ed. <br>Corrective Action: Ensure that sharps containers are wiped down with disinfectant prior to disposal. Disposal of biohazardous sharps is through a medical waste disposal contractor coordinated through EHS on the main campus or through the UTIA Safety Office on the Ag campus and at the College of Veterinary medicine. Biohazardous sharps containers are never to be disposed of in the regular trash, regardless of treatment status.
-
Is shared equipment or equipment in common areas containing propagative Risk Group 2 (RG2) agents properly secured and placarded with content owner's contact information?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: Lab Security Policy LS-004 Corrective Action: Ensure that equipment containing RG2 propagative agents in unsecured areas is locked at all times. Ensure the storage equipment has the biohazard symbol affixed to the equipment and a sign identifying the content owner's name and emergency contact number.
-
Are doors to BSL-2 labs able to be secured?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: CDC/NIH BMBL 6th ed.
Corrective Action: Ensure doors to BSL-2 labs are locked when not in use.
Chemical
-
Are there chemicals in this lab?
-
Is the chemical inventory updated within 12 months?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.1200(a)(2) or EHS procedure EC-004 (https://ehs.utk.edu/index.php/table-of-policies-plans-procedures-guides/chemical-inventories/) Corrective Action: Chemical inventory management required.
-
Are corrosive chemicals stored properly (corrosive cabinet or secondary containment)?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.1450 Corrective Action: Store corrosive chemicals in a corrosive storage cabinet or in secondary containment. Do not store corrosive chemicals directly on metal or wood surfaces. Corrosive chemicals must be segregated from incompatible chemicals.
-
Are incompatible chemicals stored appropriately?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.1450 (Appendix A)
Corrective Action: Chemicals should be stored according to hazard category and compatibility. Incompatible chemicals (e.g., acid-base, oxidizer-flammable) must be physically separated within storage areas. This can be accomplished with secondary containment. Review all chemical storage and ensure proper compatibility for storage. -
Are all chemicals adequately labeled?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.1200, 1450(h)
Corrective Action: Chemical labeling must be legible and intact on all vessels containing chemicals. Replace any missing or illegible chemical labels. -
Does the lab have a lab-specific spill response plan?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.1450 (Appendix A) Corrective Action: Develop lab-specific procedures for spill response. Emergency spill supplies, appropriate for chemicals in the lab, should be available in the lab in the event of small scale/minor risk spills and emergencies (UTK Chemical Hygiene Plan - Appendix F). Larger volume/high-risk spills should be reported to EHS (865-974-5084 or ehs_labsafety@utk.edu) for assistance and possible exposure monitoring. Inspect and stock the chemical spill response materials at regularly scheduled intervals. Chemical Hygiene Plan can be found here: (https://ehs.utk.edu/index.php/table-of-policies-plans-procedures-guides/chemical-hygiene-plans/).
-
Secondary containment available for transporting chemicals?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.1450 (Appendix A)
Corrective Action: Use bottle carriers, pans, or carts with raised sides for spill containment when moving chemicals to different buildings, locations within a building, or areas of a lab. -
Is flammable storage adequate for present quantities?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 45, FS-020 Use and Storage of Flammable and Combustible Liquids
Corrective Action: Flammable liquids storage should adhere to the following flammable storage limit guidance: You may store 2 gallons of flammable liquids outside flammable cabinets and safety cans per 100 square feet of lab unit space. You may store 4 gallons of flammable liquids per 100 square feet of lab unit space if half of it is stored inside flammable liquid storage cabinets or safety cans. You may store no more than 60 gallons of flammable liquids inside any single flammable liquid storage cabinet. -
Are household refrigerators free of flammable storage and labeled to prohibit them?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 45 Corrective Action: Flammable materials must never be stored in household refrigerators. If flammable materials must be stored under refrigeration, a flammable safe refrigerator must be used. Contact EHS (865-974-5084 or ehs_labsafety@utk.edu) for further assistance.
-
Is a first aid kit appropriate for hydrofluoric acid available in the laboratory?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.1450, (Appendix A) Corrective Action: Purchase and maintain a first aid kit appropriate for hydrofluoric acid. Purchase commercially available calcium gluconate (e.g., Calgonate) to resupply. Ensure calcium gluconate is within the expiration date.
-
Are all peroxide forming chemicals appropriately labeled and dated?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.1450; NFPA 45
Corrective Action: Certain chemicals can form peroxides after prolonged storage, making the bottle potentially shock sensitive. Record the date on the label when peroxide formers arrive in your lab. If you are unsure of the stability of any of these containers DO NOT OPEN THEM - contact EHS (865-974-5084 or ehs_labsafety@utk.edu) immediately. Always write the date received and date opened directly on the bottle. -
Is unnecessary mercury use avoided in the lab?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: EC-042 Mercury Reduction Guidelines (https://ehs.utk.edu/index.php/table-of-policies-plans-procedures-guides/mercury-reduction-guidelines/)
Corrective Action: Mercury should not be used in laboratory settings (i.e., mercury thermometers) unless integrally incorporated into laboratory equipment by a manufacturer. Contact EHS (865-974-5084 or ehs_labsafety@utk.edu) for disposal of mercury-containing devices. -
Is the chemical fume hood annual inspection current?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.1200, 1450 Corrective Action: Contact EHS (865-974-5084 or ehs_labsafety@utk.edu) to test and inspect the chemical fume hood before continuing research involving a fume hood.
-
Is the chemical fume hood functioning and used properly?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.1450(e)(3)(iii)
Corrective Action: Chemical fume hoods are used for most hazardous chemical work. They are not to be used for hazardous chemical storage. Do not block fume hood airflow. If the airflow is blocked with equipment, bottles, or unnecessary clutter, efficient capture is reduced which potentially increases exposure. Every fume hood should have a sticker on it that explains how to properly use the hood. The sash should be lowered when not in use. If the fume hood is malfunctioning or damaged, immediately stop use and report to Facilities Services (865-946-7777) and EHS (865-974-5084). -
Are there procedures in place to ensure prior approval is given to unattended experiments?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: UTK Chemical Hygiene Plan 4.7 (https://ehs.utk.edu/index.php/table-of-policies-plans-procedures-guides/chemical-hygiene-plans/) Corrective Action: Unattended experiments (reactions run overnight, reactions cooled with building-supplied water, etc.) shall have prior approval by the principal investigator. This is to ensure that all precautions have been taken to prevent accidents. As a best practice, a circulating chiller should be used to cool unattended experiments instead of building-supplied water.
PPE
-
PPE Evaluation
-
Are appropriate gloves available and required to be worn for the hazards present?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.132-139 (SUBPART I),1450 Appendix A,1030; CDC/NIH BMBL 6th ed.
Corrective Action: Select and require lab personnel to use appropriate hand protection when employees' hands are exposed to hazards such as those from skin absorption of harmful substances; infectious materials; severe cuts or lacerations; severe abrasions; punctures; chemical burns; thermal burns; and harmful temperature extremes. Contact EHS (865-974-5084 or ehs_labsafety@utk.edu) for assistance with glove selection. -
Are disposable gloves which are worn for protection from hazardous materials used appropriately (not reused, not used to touch clean common contact surfaces)?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.1450, 1030; CDC/NIH BMBL 6th ed. Corrective Action: Do not reuse disposable gloves when worn for protection from hazardous materials. Remove gloves and wash hands prior to leaving the laboratory or before touching common contact surfaces (e.g. keyboard). Use the one glove method if transporting hazardous materials outside of the lab.
-
Are appropriate lab coats available and required to be worn for the hazards present?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.1450, Appendix A,1030; CDC/NIH BMBL 6th ed. Corrective Action: Lab coats should be worn when working with hazardous materials in a laboratory. Additional protective clothing should be used when there is significant potential for skin-contact exposure to hazardous materials. The protective characteristics of this clothing must be matched to the hazard. Never wear laboratory coats outside the laboratory or into areas where food is stored and consumed.
-
Is appropriate eye and face protection available and required to be worn for the hazards present?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.132-139 (SUBPART I),1450 Appendix A,1030; CDC/NIH BMBL 6th ed.
Corrective Action: The employer shall ensure that each affected employee uses appropriate eye or face protection when exposed to eye or face hazards from flying particles, molten metal, liquid chemicals, acids or caustic liquids, infectious materials, chemical gases or vapors, or potentially injurious light radiation -
Are respirators worn in the lab?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.134 and UTK Respiratory Protection Procedure (IH-003).
Corrective Action: Contact EHS (ehs_labsafety@utk.edu or 865-974-5084) for respiratory protection applicability and program enrollment. -
Are respirators worn voluntarily (e.g. nuisance dust or particulates)?
-
Are lab members who are required to wear respirators enrolled in the respiratory protection program?
-
Regulatory Citation: 29 CFR 1910.134 and UTK Respiratory Protection Procedure (IH-003).
Corrective Action: Contact EHS (865-974-5084 or ehs_labsafety@utk.edu) for assistance. -
Have wearers reviewed Appendix D of Appendix A: Voluntary Use Respirator Information?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.134 and UTK Respiratory Protection Procedure (IH-003). Corrective action: Please complete and keep the following form on file (https://ehs.utk.edu/wp-content/uploads/2018/03/IH-003-Respiratory-Protection-AppA-Voluntary-Use.pdf).
-
Is reusable PPE (e.g. lab coats, eye and face protection, butyl gloves) kept in good condition and cleaned and stored according to manufacturer's instructions?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.132-139 (SUBPART I),1450 Appendix A,1030; CDC/NIH BMBL 6th ed. Corrective Action: Keep reusable PPE clean and stored according to the manufacturer's instructions.
Hazardous Waste
-
Does this lab generate hazardous waste?
-
Is chemical waste properly managed and labeled?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: RCRA Subtitle C; 29 CFR 1910.120; 40 CFR 262 Corrective Action: Hazardous waste collection containers must be: (1) Closed except when adding or removing waste. (2) Labeled with a complete hazardous waste label. Labels must be legible and include the contents of the container with full chemical names, no abbreviations or formulas. (3) Liquids must be stored in secondary containment bins. Fume hoods are NOT secondary containment. Secondary containment is needed in the fume hood to house hazardous waste. (4) Incompatible wastes (e.g. acid-base) must be segregated. (5) Full waste containers must be disposed of promptly.
-
Are chemicals properly disposed?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: RCRA Subtitle C; 40 CFR 262 Corrective Action: Chemicals must be disposed of following proper hazardous waste procedures. Chemicals must be properly disposed of, not evaporated via a chemical fume hood nor disposed of via the sink and sanitary sewer, without EHS (865-974-5084 or ehs_labsafety@utk.edu) approval. Allowing solvents to evaporate in the fume hood is an unacceptable disposal method.
-
Is chemical waste kept out of regular trash and glass recycling?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: RCRA Subtitle C; 40 CFR 262
Corrective Action: Chemically contaminated waste must be properly disposed of in appropriate chemical waste receptacles. Consult with EHS (865-974-5084 or ehs_labsafety@utk.edu) for disposal of empty chemical bottles. -
Is hazardous waste storage area properly labelled?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: UTK EHS Hazardous Waste Management EC-001 EC-001 (https://ehs.utk.edu/index.php/table-of-policies-plans-procedures-guides/hazardous-waste-management/).
Corrective Action: All hazardous waste should be stored in a Satellite Accumulation Area (SAA) which should be clearly marked with a sign (signs can be obtained from EHS).
Compressed Gases/Cryogens
-
Are there compressed gases/cryogens in this lab?
-
Are all compressed gases capped while stored?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 1, 63.3.1.10.1 Corrective Action: Remove the regulator and replace valve-protection cap when a cylinder is not in use. Cylinders without a regulator require a valve-protection cap.
-
Are all compressed gas cylinders and lecture bottles stored upright and properly secured?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 45, 10.1.5.1; 55, 7.1 Corrective Action: Properly secure all compressed gas cylinders and lecture bottles in an upright position. Secure all cylinders to a wall, bench or other fixed structure with a strap or chain or other suitable engineered securing device. Submit a request for the installation of a chain or anchor to Facilities Services, if needed.
-
Are incompatible compressed gas cylinders properly segregated while being stored?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 1, 63.3/1.11.2 Corrective Action: Oxygen and flammable gases segregation: Store cylinders at least 20 feet apart, store cylinders in separate rooms or separate cylinders with a 30-minute fire-rated barrier. Other incompatible gases (eg. toxic and corrosive gases) should be segregated and not stored together.
-
Are all compressed gas cylinders and lecture bottles properly labeled?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 1, 63.1.8.1, OSHA 29 CFR 1910.1200, 1450 Corrective Action: Properly label gas cylinders/lecture bottles
-
Is storage of compressed gas cylinders within allowable limits?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 45, 10.1.6.5; 45, 10.1.6.4 Corrective Action: Reduce the number of compressed gases to below the maximum allowable quantity (MAQ). Remove spare cylinders from the laboratory. Return cylinders to gas supplier or store in approved gas storage outside of the lab.
-
Are toxic gases properly used and stored?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 45, 10.1.4 Corrective Action: Toxic gases must be used in a chemical fume hood or a ventilated gas cabinet. If a gas cabinet is not available, purchase a gas cabinet or remove gas from the lab.
-
Is tubing used to dispense compressed gases compatible and properly secured?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 45 Corrective Action: Properly secure tubing. Ensure tubing is compatible with gas being dispensed. Contact EHS (974-5084 or ehs_labsafety@utk.edu) for assistance.
-
Are cryogens properly used and stored?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Corrective Action: Move cryogens to a well-ventilated area or purchase an oxygen monitoring system. Contact EHS at [865-974-5084 or ehs_labsafety@utk.edu] for assistance in selecting an appropriate oxygen monitor.
-
Are proper safety controls in place while using cryogens?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Corrective Action: Follow cryogen use guidelines on the EHS website (https://ehs.utk.edu/index.php/table-of-policies-plans-procedures-guides/compressed-gases-and-cryogens/). Make sure appropriate PPE is stocked in the lab.
-
Is the cryogen dispensing apparatus appropriate?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Corrective Action: Purchase a metal dispensing hose for dispensing cryogens. Provide training for dispensing from the bulk system (JIAM, Physics, and Dabney-Buehler).
Electrical Safety
-
Are the electrical cords and plugs on all appliances and equipment in good condition?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 1, 11.1.2.2
Corrective Action: Electrical cords and plugs on appliances and equipment must be maintained in good condition to minimize the risk of shock or fire. If the equipment is hard-wired, submit a work order to repair the electrical cord to Facilities Services. If the equipment is plugged into a receptacle, contact the manufacturer or your departmental or college electric shop for repair. -
Are aisles clear of electrical cords and cables?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 1, 14.4.1 Corrective Action: Cords/cables must be protected with a cable bridge when they cross aisles to avoid tripping hazards and prevent damage to the cords or cables. Relocate cords/cables or purchase a cable bridge.
-
Are all extension cords/power strips located away from water sources, chemicals or heat sources?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: OSHA 1910.334(a)(4) Corrective Action: Move electrical cords/power strips away from water, chemicals, or heat sources.
-
Are there 36 inches of clearance in front of all electrical panels?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: OSHA Standard 1910.303 (g) Corrective Action: Maintain 36 inches of clearance in front of electrical panels. Remove obstructions from in front of an electrical panel.
-
Are all extension cords and power strips plugged directly into receptacles? (Not piggy-backed.)
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 1, 11.1.4.2 Corrective Action: Extension cords and power strips must be plugged directly into electrical receptacles. Connecting extension cords and power strips in series increase the electrical resistance and heat generation, which can lead to equipment damage or fire.
-
Are extension cords used only for immediate or temporary use (less than 90 days)?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 1, 11.1.5.6 Corrective Action: Extension cords can be used only for immediate or temporary use (less than 90 days). Relocate equipment so that it can be plugged directly into a receptacle. If this is not possible, contact Facilities Services to request the installation of additional receptacles.
-
Are GFCI receptacles present within 6 feet of water sources?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.304
Corrective Action: Electrical receptacles located within 6 feet of a water source (i.e. sink) are required to have ground-fault circuit interrupter (GFCI) protection. Submit a work order to Facilities Services to install GFCI protected receptacles. -
Are covers present on all receptacles and electrical equipment?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 1, 11.1.8
Corrective Action: All receptacles, switches, electrical panels, junction boxes, and other electrical equipment must be covered. Submit a request to correct deficiency to Facilities Services.
Emergency Equipment
-
Is emergency equipment present?
-
Is an eyewash readily available (as necessary)?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: ANSI/ISEA Z358.1 Corrective Action: An eyewash is required when there is a possibility of eye exposure to corrosive materials. Eyewashes must be located within 10 seconds walking time from the location of the hazard. Submit a request for the installation of eyewash to Facilities Services.
-
Is eyewash clear of obstructions?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: ANSI/ISEA Z358.1 Corrective Action: The area around eyewashes and the path of travel to the eyewash must be free of obstructions at all times. Remove obstructions from around the eyewash and discuss with the lab group.
-
Is eyewash tested weekly?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: ANSI/ISEA Z358.1 Corrective Action: Test the eyewash(es) weekly to ensure the eyewash is functioning properly, clear the supply line of any sediment buildup, and minimize microbial contamination due to stagnant water. Eyewash log can be found here (https://ehs.utk.edu/wp-content/uploads/2018/10/HM-020-Safety-Showers-and-Eyewashes-AppA-Eyewash-Test-Log.pdf).
-
Is a safety shower readily available?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: ANSI/ISEA Z358.1 Corrective Action: A safety shower is required when there is a possibility of skin exposure to corrosive materials. Safety showers must be located within 10 seconds of walking time from the location of the hazard. Submit a request for the installation of a safety shower to Facilities Services.
-
Is safety shower clear of obstructions?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: ANSI/ISEA Z358.1 Corrective Action: The area around safety showers and the path of travel to the shower must be free of obstructions at all times. Remove obstructions from around the safety shower and discuss with the lab group.
-
Has safety shower been inspected within the past year?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: ANSI/ISEA Z358.1 Corrective Action: Safety showers must be tested by Facilities Maintenance annually to ensure that the shower is functioning properly and there is sufficient flow. Submit a request to Facilities Services to have the safety shower tested.
-
Are chemical/gas detection or alarm systems calibrated and tested regularly?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: IFC Corrective Action: Chemical or gas detection/alarm systems must be calibrated and/or tested according to the manufacturer's recommended schedule and procedure. Alarm systems must be tested at least annually. If the alarm system is connected to the building's fire alarm system, Facilities Services must be notified prior to testing. Contact EHS (865-974-5084 or ehs_labsafety@.utk.edu) for assistance.
Fire Safety
-
Are areas around heat sources kept clear of combustibles?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 45, 11.2.7
Corrective Action: Store combustible materials and flammable liquids away from heat or flame (i.e. Bunsen burners, hot plates) to reduce the risk of fire. When open flame operations can not be conducted in a shielded enclosure, do not conduct work under shelving, cabinets, or other overhangs. -
Are the aisle, egress, fire alarm pull stations, fire extinguishers, and sprinklers unobstructed?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: IFC 903.3, 906.6, 907.4 1003, 1031 Corrective Action: Laboratory occupants must always have an unobstructed pathway to allow rapid egress in the event of an emergency. Emergency equipment such as fire extinguishers, fire alarm pull stations, and fire suppression sprinkler heads must always be unobstructed to permit access and allow proper operation. Remove obstructions.
-
Are fire doors in good condition?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 1, 12.4.6.9.
Corrective Action: Fire doors must be maintained in good condition to provide proper fire separation from non-laboratory areas. Submit a request to repair the fire door to Facilities Services. -
Are fire doors kept closed?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 1, 12.4.6.2.2. Corrective Action: The fire door must be kept closed and latched to provide proper fire separation from non-laboratory areas. Close fire doors.
-
Are fire rated partitions (wall, floor, ceiling) free from penetrations?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 1, 12.3.3.1. Corrective Action: The walls, floor, and/or ceiling in this laboratory are fire-rated partitions and cannot be penetrated. Penetrations must be properly sealed to provide proper fire separation. Submit a work order for repairs to Facilities Services.
-
Are fire extinguishers present (as required)?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 1, 13.6.3.1.2
Corrective Action: All laboratories using flammable chemicals are required by the State Fire Code to have a fire extinguisher in the laboratory or within 50 feet of the laboratory. Submit a request to have the missing fire extinguisher replaced to EHS (865-974-5084 or safety@tennessee.edu). -
Are fire extinguishers mounted?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 1, 13.6.3.1.3.8 Corrective Action: Submit a request to install a mounting bracket for the fire extinguisher to EHS Fire and Life Safety group (865-974-5084 or safety@tennessee.edu).
-
Are fire extinguishers in good condition, charged, and recently inspected?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 1, 13.6.3.1.2
Corrective Action: Submit a request to have defective or discharged extinguisher(s) replaced to EHS Fire and Life Safety group. -
Are fire extinguishers appropriate for hazards?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 1, 13.6.2
Corrective Action: Fire extinguisher type is determined by the hazards in your laboratory. Submit a request to replace fire extinguisher(s) with proper type and size to EHS (865-974-5084 or safety@tennessee.edu). -
Are all ceiling tiles in place?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 13, 3.3.3 Corrective Action: Missing ceiling tiles can affect proper sprinkler or smoke detector activation. EHS will submit a Facilities Services request to have missing ceiling tiles repaired/replaced.
-
Are all sprinklers free from obstructions due to storage?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NFPA 1,10.18.3.2
Corrective Action: To allow for proper water distribution, sprinklers must never be obstructed. Remove storage within 18 inches of sprinkler heads.
General
-
General Safety Evaluation
-
Is a hand washing sink including soap and hand towels or approved alternative methods available?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.1450; CDC/NIH BMBL 6th ed.
Corrective Action: Ensure hand washing is not discouraged because hand washing facilities are not adequate. -
Is the laboratory housekeeping satisfactory?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.22; CDC/NIH BMBL 6th ed.
Corrective Action: An organized work environment is vital when working with hazardous materials. Clutter can lead to falls, chemical spills, exposures and can block emergency exits. Poor housekeeping can hide contamination and contribute to overall exposure. Keep all work areas clear and support custodial staff by managing trash properly. General housekeeping should be actively maintained, including disposal of garbage and unnecessary equipment. -
Are laboratory work areas free of food and drink?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.1450; 29 CFR 1910.1200; CDC/NIH BMBL 6th ed.
Corrective Action: Remove all food and drink from laboratory work areas. -
Are 'No Food/Drink' signs conspicuously posted on refrigerators, freezers, or other applicable equipment?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.1450
Corrective Action: Food and beverages are not to be stored in refrigerators, freezers, or other applicable lab equipment. Post appropriate 'No Food or Drink' signage on appropriate equipment. -
If available, are first aid kit items in date?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.151
Corrective Action: Inspect and replenish first aid kit supplies on a scheduled basis.
Documentation
-
Documentation Evaluation
-
Is a current Chemical Hygiene Plan available?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: OSHA 29 CFR 1910.1450
Corrective Action: A Chemical Hygiene Plan is required for all laboratories that use hazardous chemicals. The Chemical Hygiene Plan must be reviewed annually and updated as needed. Prepare or update laboratory-specific Chemical Hygiene Plan. Contact EHS (865-974-5084 or ehs_labsafety@utk.edu) for assistance. https://ehs.utk.edu/index.php/table-of-policies-plans-procedures-guides/chemical-hygiene-plans/ -
Has general lab safety training been completed?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: OSHA; 29 CFR 1910.1200, 1450
Corrective Action: All personnel working with or around hazardous chemicals or other laboratory hazards are required to be trained. Personnel must complete EHS-required general lab safety training prior to beginning lab work. Additionally, each laboratory supervisor (PI, manager, instructor) is ultimately responsible for ensuring those working under their supervision are trained and understand the specific and unique hazards their work presents (lab-specific training). Attendance and content of all training must be documented. Online training can be found here (https://ehs.utk.edu/index.php/training/). Training forms can be found here (https://ehs.utk.edu/index.php/training/training-forms/). -
Has hazardous waste training been taken in the past 12 months?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1200; 40 CFR 262 Corrective Action: All researchers who handle hazardous chemicals in the teaching or research labs should complete hazardous waste training every 12 months. https://ehs.utk.edu/index.php/training/
-
Has laboratory hazard assessment been performed and proper PPE identified?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.1450; 1910.132-139 (SUBPART I); CDC/NIH BMBL 6th ed. Corrective Action: A hazard assessment (type and volume of hazards present) will dictate the protective equipment needs of the laboratory. Laboratories must keep a selection of appropriate gloves, safety glasses, splash goggles, aprons, and lab coats readily available, as needed. Glove permeation and selection guides should be consulted. Perform a hazard assessment to determine the necessary protective equipment for the laboratory. Contact EHS (865-974-5084 or ehs_labsafety@utk.edu) for assistance with hazard assessment and PPE determination or review CHP (https://ehs.utk.edu/index.php/table-of-policies-plans-procedures-guides/chemical-hygiene-plans/).
-
Are Safety Data Sheets (SDS) available?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: OSHA 29 CFR 1910.1200, 29 CFR 1910.1450
Corrective Action: OSHA requires that safety data sheets (SDS/MSDS) for chemicals used in the lab be available and accessible to all lab members. Access can be provided via paper copies, electronic copies, or the internet. SDS/MSDS are available from the chemical manufacturer and are included with chemical shipments. Laboratories are encouraged to maintain printed SDS/MSDS for their acutely toxic chemicals and keep them in an accessible location. This ensures access during electric, network, or server interruptions. Ensure SDS/MSDS for chemicals used in the lab are available and accessible. More information can be found in the CHP (https://ehs.utk.edu/index.php/table-of-policies-plans-procedures-guides/chemical-hygiene-plans/). -
Are standard operating procedures (SOPs) available?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: OSHA 29 CFR 1910.1450
Corrective Action: SOPs are required for work involving hazardous materials in the laboratory. SOPs must include a summary of the hazards, the controls needed to minimize exposures, and safe handling/storage procedures. Prepare SOPs for work involving hazardous materials and review procedures with lab members. Contact EHS (865-974-5084 or ehs_labsafety@utk.edu) for assistance or an SOP template (https://ehs.utk.edu/wp-content/uploads/2020/02/LS-020-CHP-AppA-LabSpecific-Sec04.1-SOP-Form.pdf). -
Biosafety training
-
Are initial training records available for all personnel working in registered research labs, diagnostic labs, the Forensic Anthropology Center, and in other capacities that requiring Biosafety training?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.1030; CDC/NIH BMBL 6th ed. Corrective Action: Complete initial training, which can be accessed on the Biosafety training web page (https://biosafety.utk.edu/biosafety-program/training/).
-
Are refresher training records that include BBP training available and/or are there plans for completing refresher training in this audit cycle?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.1030; CDC/NIH BMBL 6th ed.
Corrective Action: Ensure all employees have completed Biosafety refresher training annually (https://biosafety.utk.edu/biosafety-program/training/). -
Has lab-specific Biosafety training been completed by lab personnel?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: 29 CFR 1910.1030; CDC/NIH BMBL 6th ed.
Corrective Action: Ensure all lab personnel have completed lab-specific Biosafety training and/or the Chemical Hygiene Plan Orientation Checklist (https://ehs.utk.edu/index.php/table-of-policies-plans-procedures-guides/chemical-hygiene-plans/). -
Does the laboratory use a shipping designee for biological/dry ice samples that has had training within the past two years?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: IATA Dangerous Goods Regulations: 49 CFR 171-180.
Corrective Action: Ensure all employees have completed dangerous goods shipping training or have the awareness to contact EHS (865-974-5084 or ehs_labsafety@utk.edu) for shipping assistance if shipping training is not current.
Other Findings
-
Other Comments/Corrective Actions:
-
Other Comments/Corrective Actions:
Miscellaneous
-
Miscellaneous?
-
What section?
- Physical Hazards
- Animal
- Controlled Substance
- General
Physical Hazards
-
Are laboratory personnel prohibited from using mechanical equipment while wearing loose clothing and unsecured long hair?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: OSHA 29 CFR 1910 Subpart I.
Corrective Action: Wear properly fit clothing and secure long hair so that they can not be entangled in equipment. Discuss with laboratory personnel and include in future training. -
Are manufacturer provided guards present on mechanical equipment and/or safety interlocks functional?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: OSHA 29 CFR 1910.212. Corrective Action: Mechanical guards removed and/or safety interlocks inactivated. Guards on mechanical equipment isolate the hazards associated with moving parts. Reaffix any manufacturer-provided guards.
Animal
-
Are cages marked with appropriate hazard stickers?
-
Risk
-
Warning labels may be obtained from EHS. If hazardous materials are used in animal cages, it must be noted with appropriate warning labels on the front of the cage. Cages need to be properly marked with biohazard sticker and the specific agent.
-
Is pest control regularly scheduled?
-
Risk
-
Pest Control Program Needed: Pest Control Programs are designed to prevent, control, or eliminate the presence of or infestation by pests are essential in an animal environment. A regularly scheduled and documented program of control and monitoring should be implemented.
-
Are anesthetic gas delivery systems properly configured to scavenge or minimize anesthetic gas vapors?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Regulatory Citation: NIOSH Recommended Exposure Limits and ACGIH Threshold Limit Values for anesthetic gases. Corrective Action: Properly configure the gas system to scavenge or minimize anesthetic gas vapors. Contact EHS at 865-974-5084 or ehs_labsafety@utk.edu for assistance.
General
-
Are all lab personnel wearing appropriate laboratory attire (e.g., long pants and solid, closed-toed shoes)?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
29 CFR 1910.1450 Appendix A: Replace shorts/open toe shoes with long pants and closed toe shoes. The shoes should cover the toes, sides, heels and most of the tops of the feet and not be made of absorbent material.
-
Have all modifications to building infrastructure in laboratory been approved?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
NFPA 1, 1.3.6.2: All modifications (repairs, renovations, alterations, reconstruction, change of occupancy) to building infrastructure and hazard control devices (e.g., chemical fume hood, biosafety cabinet, etc.) must be reviewed and approved by Facilities Services and EHS to ensure adherence to safety, building, and fire code requirements.
-
Is absorptive furniture kept out of the laboratory?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
NRC Prudent Practices in the Laboratory 9.B.5.2: Replace any fabric or porous furniture found in laboratory work area with non-fabric alternatives that allow for effective spill clean up and decontamination.
Controlled Substances
-
Have controlled substances been reported to the Office of Research?
-
Risk
- Minor finding: Complete w/in 15 business days
- Major finding: Complete w/in 5 business days
- Serious finding: Stop Work and Fix
-
Corrective Action: Report ownership of controlled substances to the Office of Research for compliance guidance (Office of Research - research@utk.edu or 865-974-9918).
-
Have controlled substances been entered into chemical inventory?
-
Corrective Action: Enter controlled substances into chemical inventory.
Findings Closure
-
Has the report been escalated per the LSS process?
-
Please explain in detail why it was escalated.