Title Page
-
Site conducted
-
Facility工厂:
-
Audit Date & Time审核日期&时间:
-
Attendees参与者:
-
Auditor审核员:
-
Location地址:
Facility Review
RECEIVING AND STORAGE
-
1.Whether the brand has a vendor selection system; whether the vendor selection system clearly defines the selection standards, review and evaluation procedures;企业是否建立并执行物料供应商遴选制度;物料供应商遴选制度是否明确物料供应商的遴选、退出标准以及审核、评价程序;
-
2. Are their established requirements and/or certifications required for all subcontractors and suppliers? 所有分包商和供应商是否都需要审核和/或认证?-EN
-
3.Does the vendor audit their raw material suppliers? 供应商是否对原材料供应商进行审核?-EN
-
4.Whether the vendor keep relevant information/file when auditing in accordance with the vendor selection system; 企业按照物料供应商遴选制度对物料供应商进行审核时是否留存相关资料;
-
5.Does the company update the material vendor information and keep the database latest?企业是否及时对合格物料供应商档案信息进行更新,确保物料供应商档案处于最新状态。
-
6.Whether the enterprise evaluate material suppliers on a regular basis or major changes in the production conditions of material suppliers, whether to take corresponding measures according to the evaluation results, and whether to keep evaluation and processing records; 企业是否定期或者在获知物料供应商生产条件发生重大变化时对物料供应商进行评价,是否按照评价结果采取相应措施,是否留存评价和处理记录;
-
7.Does the QA leader track and evaluate the material vendors management?质量管理部门负责人是否根据物料供应商相关管理制度定期评价物料供应商;
-
8.Whether the enterprise has established a list of qualified material suppliers based on the audit and evaluation results; whether the list of qualified material suppliers includes the name, address and contact information of the material supplier, as well as the name of the material, quality requirements, and the name of the manufacturer; 企业是否根据审核评价结果建立合格物料供应商名录;合格物料供应商名录是否包括物料供应商名称、地址和联系方式,以及物料名称、质量要求、生产企业名称等内容;
-
9.Whether the company has identified key raw material suppliers, whether it has conducted key audits on them, whether it has clarified the situation where key raw material suppliers need to conduct on-site audits, and implemented them in accordance with regulations; 企业是否明确关键原料供应商,是否对其进行重点审核,是否明确关键原料供应商需要进行现场审核的情形,并按照规定执行;
-
10. Is there a contract between company and material vendors? Does it include the QA standard and accept standard?企业是否与物料供应商签订采购合同,是否在合同中明确物料验收标准和双方质量责任。
-
11.Is there the check or validation SOP to identify each step, process, record and report? 企业是否建立并执行检验管理制度;检验管理制度是否明确与检验相关的职责分工、程序、记录和报告要求等内容;
-
12.Does the QC unit have written procedures to approve or reject all component (ingredient)s, containers, closures, in-process materials, packaging material, labeling, and finished products?QC部门是否有书面程序来批准或拒绝所有成分(成分)、容器、封盖、中程材料、包装材料、标签和成品?-EN
-
13.Does the QC unit approve all procedures and specifications impacting the identity, strength, quality, and purity of the product.QC部门是否批准所有影响产品鉴别、强度、质量和纯度的程序和规范。-EN
-
14.Whether the enterprise conducts review on raw materials, purchased semi-finished products, and internal packaging materials before material procurement. 企业是否在物料采购前对原料、外购的半成品、内包材实施审查。
-
15.Does the company identify clearly for the quality validation method which match the government standard?企业采用非检验方式作为质量控制措施的,是否明确质量确认方式和要求;采用检验方式作为质量控制措施的,检验项目、检验方法和检验频次是否符合化妆品注册、备案资料载明的技术要求;
-
16.Are there any quality control requirement for the RM, inner components, semi finish goods and finish goods which should follow government standard and tech demand?企业是否制定原料、内包材、半成品以及成品的质量控制要求;质量控制要求是否符合强制性国家标准和技术规范;
-
17.Whether the enterprise distinguishes whether the raw materials specified by the entrusting party are prohibited raw materials, new raw materials without registration or filing; 企业是否分辨委托方指定原料是否属于禁用原料、未经注册或备案的新原料;
-
18.Whether the enterprise uses restricted raw materials beyond the scope of use and restrictions; 企业是否超出使用范围、限制条件使用限用原料;
-
19.Whether the raw materials used by the enterprise purchased semi-finished products, and inner packaging materials meet the requirements of laws and regulations, mandatory national standards, and technical specifications. 企业使用的原料、外购的半成品、内包材是否符合法律法规、强制性国家标准、技术规范的要求。
-
20.Whether the enterprise whose material name and supplier name are marked with a code has formulated a code management procedure, and whether it has formulated a material and supplier name code comparison table; whether the raw material code clearly specifies the corresponding raw material standard CN name; 物料名称、供应商名称用代码标示的企业是否制定代码管理规程,是否制定物料、供应商名称代码对照表;原料代码是否明确对应的原料标准中文名称;
-
21.Whether the enterprise has established a list of raw materials, outsourced semi-finished products, and internal packaging materials, whether it has clearly defined the complsition of raw materials and outsourced semi-finished products, and whether it has retained the necessary raw materials, outsourced semi-finished products, and internal packaging material quality and safety related information; 企业是否建立原料、外购的半成品以及内包材清单,是否明确原料和外购的半成品成分,是否留存必要的原料、外购的半成品、内包材质量安全相关信息;
-
22. Whether the enterprise has established and implemented a Raw material&component review system; 企业是否建立并执行物料审查制度;
-
23.Are there written procedures for the receipt, identification, storage, handling, sampling, testing and approval of components (ingredients),packaging materials, and Labels?对包材(配件)、包装材料和标签的接收、标识、储存、处理、取样、测试和批准有书面程序吗? -EN
-
24.Whether the enterprise has established and implemented material acceptance procedures, and whether the acceptance criteria and acceptance methods are specified; whether the material acceptance procedures require the retention of material qualification certificates, delivery tickets, etc.; whether imported raw materials that require inspection and quarantine require retention of relevant certificates; 企业是否建立并执行物料验收规程,是否明确验收标准和验收方法;物料验收规程是否要求留存物料合格出厂证明文件、送货票证等;需要检验、检疫的进口原料是否要求留存相关证明;
-
25.Whether the enterprise has established and implemented a material purchase inspection record system; 企业是否建立并执行物料进货查验记录制度;
-
26.Whether the enterprise has truthfully recorded the material, the inventory quantity of the products and the changes of receiving, issuing, returning... 企业是否如实记录物料和产品的库存数量和接收、发放、退回等变动情况。
-
27. Whether the enterprise has established and implemented a material release management system; whether the standards for material release approval, division of responsibilities, and other requirements are clarified; .企业是否建立并执行物料放行管理制度;是否明确物料批准放行的标准、职责划分等要求;
-
28.Does the QA leader audit and track the material or products released?质量管理部门负责人是否承担物料和产品的放行审核工作,并保证审核工作可追溯;
-
29. Whether the materials used for production are released according to regulations. 用于生产的物料是否按照规定放行。
-
30.Are COA’s required for component (ingredient) approval?组件(成分)的批准需要COA吗?-EN
-
31.Are COA's obtained for packaging components and labels? 是否收集包装部件和标签的COA ?-EN
-
32.Are components (ingredients), containers and closures withheld from production until released by Q.C.? 包材(配件)、外箱和瓶盖是否在qc放行前不放行?-EN
-
33.Are labels held in quarantine until issued to the production area?标签是否被隔离,直到签发到生产区?-EN
-
34.. Do incoming fragrance oils have unique handling requirements, including inspection, testing, and release criteria.收货的香精是否有特殊的处理要求,包括检查,测试和放行标准。-EN
-
35. Is there a representative sized sample taken?是否有具有代表性的样本被取样?-EN
-
36. Are the component (ingredient) containers cleaned before sampling? 取样前组件(成分)容器是否清洁?-EN
-
37. Are the components (ingredients) sampled and resealed in a manner and in an area designed to prevent contamination?组件(成分)取样和重新密封操作的方式/区域设计是否都是防止污染的?-EN
-
38. Are component (ingredient) samples labeled with lot number, container, the date and the person who collected the sample?组件(成分)样品是否标有批号、容器编号、日期和收集样品的人?-EN
-
39. Are the components (ingredients) tested by at least one identity test?组件(成分)是否经过至少一次识别测试?-EN
-
40.Are representative samples of each lot of raw material pulled and tested/examined for physical, chemical and/or microbiological characteristics as specified?每批原料的代表性样品是否按照规定进行物理、化学和/或微生物特性测试/检验?-EN
-
41.Are components (ingredients) status labeled or held in a quarantine area until released for use?包材(配件)状态是否有标签标识或保存在隔离区直至放行使用? -EN
-
42. Whether the materials are stored in accordance with the specified conditions, whether they are classified and stored in batches according to pending inspection, qualified, unqualified, etc., and clearly marked; Whether the enterprise has marked the name of the material (the raw material should be marked with the CN name of the raw material standard) or code, supplier name or code, production date or batch number, service life, storage conditions and other information; 物料是否按照规定的条件贮存,是否按照待检、合格、不合格等分批分类存放,并明确标示;企业是否标示物料名称(原料应当标识原料标准中文名称)或者代码、供应商名称或者代码、生产日期或者批号、使用期限、贮存条件等信息;
-
43. Whether the product is stored in accordance with the specified conditions, whether it is stored in batches according to pending inspection, qualified, unqualified, etc., and clearly marked; whether the product name, batch number, service life, qualified pending inspection status, etc. are marked; 产品是否按照规定的条件贮存,是否按照待检、合格、不合格等分批分类存放,并明确标示;是否标示产品名称、批号、使用期限、合格待检状态等信息;
-
44. Is there a quarantine system for rejected component (ingredient)s and packaging materials designed to prevent their use?对不合格的组件(成分)和包装材料是否有隔离系统以防止其使用? -EN
-
45. Are bagged or boxed raw materials stored off the floor?是否有袋装或盒装的原材料储存在地板上? -EN
-
46. Is FIFO used for component (ingredient)s, containers and closures?先进先出是否用于组件(原料)、容器和盖子?-EN
-
47.Whether the enterprise formulates production and picking operation procedures; 企业是否制定生产领料操作规程;
-
48.Whether the requisition and confirmation records of materials to be used in production meet the requirements of production instructions.生产待使用物料领用和确认记录是否符合生产指令的要求。
-
49. Whether the requisitioners check and receive materials one by one according to the requirements of the product formula in the production instructions, whether they complete the requisition form and keep relevant records, whether they check the packaging and label information of the received materials and the qualified release confirmed by the quality management personnel.领料人是否按照生产指令中产品配方的要求逐一核对领取物料,是否完整填写领料单并保存相关记录,是否对所领用物料的包装、标签上的信息以及质量管理人员确认合格放行情况等进行核对。
-
50. Is there a system to identify expired component (ingredient)s and quarantine them to prevent their use?是否有一个系统来识别过期成分并隔离它们以防止使用?-EN
-
51. Are the unqualified materials clearly marked and are they stored in a special area; whether the enterprise promptly disposes of unqualified materials that have exceeded the shelf life. 不合格物料是否有清晰标识,是否在专区存放;企业是否及时处理超过使用期限等的不合格物料。
-
52.Whether the enterprise has established and implemented procedures for non-conforming materials disposition; 企业是否建立并执行不合格物料处理规程;
-
53. Are fragrance oils reinspected prior to use to ensure oil still meets standard?香精在使用前是否重新检查以确保仍然符合标准?-EN
-
54.Whether the enterprise has established the rules for keeping samples of key raw materials; 企业是否建立关键原料留样规则;
-
55.Whether the enterprise has established a catalog of key raw materials; whether it has retained samples of key raw materials according to regulations, and kept records of the samples; 企业是否建立关键原料目录;是否按规定对关键原料留样,并保存留样记录;
-
56.Whether the label of the reserved sample complies with the regulations and ensures traceability; whether the quantity of the reserved sample meets the requirements of raw material quality inspection; whether the reserved sample is sealed and stored according to the specified conditions. 留样标签是否符合规定,保证可追溯;留样数量是否满足原料质量检验的要求;留样是否密封并按规定条件贮存。
WEIGH AREA(EN)
-
1.components (ingredients)?是否有书面流程来规范称重,标记(中间容器)和记录组件(成分)重量等操作?-EN
-
2. Is the reproduction of the master production record, checked for accuracy, dated and signed?是否有复核人对称重记录的精度,日期和签名进行检查?-EN
-
3. Is the intermediate component (ingredient) container labeled with; name or code, control number, weight, and batch for which it is designated?中间组分(成分)容器上是否标有:名称或代码,控制编号,重量,指定的批次?-EN
-
4. Is the component (ingredient) weighing examined by a second person for component release, for correct weight as in production record, and for correctly labeled container?组件(成分)的称重是否由第二人进行检验,以确保组件放行,确保生产记录中的重量正确,确保容器标签正确?-EN
-
5. Is the weigh area a controlled environment to prevent contamination of open containers of components (ingredients)?称重区域是否为受控环境,以防止组件(配料)的开箱污染?-EN
-
6. Are the intermediate containers constructed of appropriate materials to prevent contamination and to facilitate cleaning?中间容器是否采用适当的材料来防止污染和便于清洗?-EN
-
7. Are batch adjustments documented as part of the batch record?批调整是否记录为批记录的一部分?-EN
CALIBRATION
-
1.Whether the enterprise has established a laboratory suitable for the beauty variety, quantity and production license items; 企业是否建立与生产的化妆品品种、数量和生产许可项目等相适应的实验室;
-
2.Are adequate laboratory facilities available to the QC unit for testing and approval or rejection of all component (ingredient)s, in-process materials, packaging material, labeling, and finished product?QC部门是否有足够的实验室设施来测试和批准或拒绝所有的组分(成分),过程材料,包装材料,标签和成品?-EN
-
3.Whether the enterprise has the ability to do microbial inspection items such as the total number of colonies, the total number of molds and yeasts; 企业是否具备菌落总数、霉菌和酵母菌总数等微生物检验项目的检验能力;
-
4.Whether the laboratory can meet the testing needs, including testing environment, testing personnel, testing facilities, equipment, instruments and reagents, culture medium, standards, etc; 实验室的检测环境、检验人员以及检验设施、设备、仪器和试剂、培养基、标准品等是否可以满足检验需要;
-
5.When an enterprise entrusts an inspection and testing agency to inspect heavy metals, pathogenic bacteria, and other safety risk substances specified in the standards implemented by the product, whether the entrusted inspection and testing agency has the qualifications and inspection capabilities for the corresponding inspection items; whether the entrusted inspection agreement or relevant documents clearly define Inspection items, inspection basis, inspection frequency and other requirements. 企业委托检验检测机构检验重金属、致病菌和产品执行的标准中规定的其他安全性风险物质时,受托检验检测机构是否具有相应检验项目的资质和检验能力;委托检验协议或者相关文件是否明确了检验项目、检验依据、检验频次等要求。
-
6.Whether the enterprise has established and implemented a laboratory management system; whether it has made clear regulations on the management of equipment, instruments, reagents, culture media, and standards to ensure that the test results are true, complete, and accurate; 企业是否建立并执行实验室管理制度;是否对设备、仪器和试剂、培养基、标准品的管理作出明确规定,保证检验结果真实、完整、准确;
-
7.Whether the enterprise has established a list of laboratory equipment and instruments; whether the equipment and instruments have unique numbers and obvious status identification; 企业是否建立实验室设备、仪器清单;设备、仪器是否设置唯一编号并有明显的状态标识;
-
8.Whether the enterprise calibrates or verifies, uses, cleans, and maintains laboratory equipment and instruments in accordance with regulations to ensure the normal operation of laboratory equipment and instruments; 企业是否按规定对实验室设备、仪器进行校准或者检定、使用、清洁、维护,保证实验室设备仪器正常运行;
-
9.Whether the enterprise implements effective management of the procurement, storage, preparation, labeling, use, scrapping and expiration date of reagents, culture media, and standard products used in the laboratory. 企业是否对实验室使用的试剂、培养基、标准品等的采购、贮存、配制、标识、使用、报废和有效期等实施有效管理。
-
10.Are there written procedures for calibration of weigh scales and other measurement instruments?对秤和其他测量仪器是否有书面的校准程序?-EN
-
11. Whether the enterprise has made a verification or calibration plan for key weighing instruments, measuring tools, instruments and instruments, and whether the verification or calibration is carried out regularly according to the plan. 企业是否制定关键衡器、量具、仪表和仪器检定或者校准计划,是否根据计划定期进行检定或者校准。
-
12.设备校准的测量仪器清单?-EN
-
13. Is there documentation of periodic calibration of the measurement instruments showing that the instruments were calibrated on schedule to a preset tolerance and with a traceable reference standard?是否有定期校准仪器的文件,显示仪器在预定的公差和可追溯的参考标准下按时校准?-EN
INGREDIENT WATER SYSTEM
-
1.Whether the amount of water used for production meets the production requirements;企业生产用水的水量是否满足生产要求;
-
2.If the production water supply is centralized, whether the enterprise can provide the source of the production water certification materials; If the water used for production is small centralized water supply or distributed water supply, whether the enterprise can provide an annual report on the inspection of the water used for production by qualified inspection and testing institutions.生产用水为集中式供水的,企业是否可以提供生产用水来源证明资料;生产用水为小型集中式供水或者分散式供水的,企业是否能够提供每年由取得资质的检验检测机构对生产用水进行检测的报告。
-
3.Whether the enterprise has formulated process water quality standards and process water management regulations according to product quality requirements;企业是否根据产品质量要求制定工艺用水质量标准、工艺用水管理规程;
-
4. Are there written procedures for the operation, cleaning, maintenance and sanitization of the ingredient water system?原料水系统是否有书面的操作、清洁、维护和消毒程序? -EN
-
5, Whether the company has established and implemented the system of regular cleaning, disinfection, monitoring and maintenance of the water treatment system, and whether it has implemented corresponding measures according to the system and kept relevant records.企业是否建立并执行水处理系统定期清洁、消毒、监测、维护制度,是否按照制度落实相应措施,并留存相关记录。
-
6.Whether the design, installation, operation and maintenance of the enterprise water production, water storage and transportation system can ensure that the process water meets the quality standard requirements.企业制水、水贮存及输送系统的设计、安装、运行、维护是否可以确保工艺用水达到质量标准要求。
-
7. Is the ingredient water system validated and follow USP (FDA) guidlines for Purified Water?原料水系统是否经过验证并遵循USP (FDA)的纯化水指南? -EN
-
8. Is there a diagram of the water system showing all sample points and use points?是否有水系统的图表显示所有的样本点和使用点?-EN
-
9. A. Is the ingredient water system free of deadlegs and constructed of material to prevent contamination ?原料水系统是否没有死角,是否采用防止污染的材料建造 ?-EN
-
10. Is there an ongoing water quality monitoring program with alert and action limits?是否有持续的水质监测计划,并有警戒和行动限制? -EN
-
11.. Whether the enterprise shall regularly monitor the quality of process water according to the process water management regulations to ensure that it meets the production quality requirements.企业是否按照工艺用水管理规程对工艺用水水质进行定期监测,确保符合生产质量要求。
PROCESSING AREA
-
1. Are there written production and process control procedures approved by the QC unit?是否有经QC部门批准的书面的生产和过程控制程序?-EN
-
2.Are product production processes (manufacturing instructions / processes) validated by both the factory and R&D?产品生产过程(生产说明书/工艺单)是否经过工厂和研发部门的验证?-EN
-
3.企业是否建立并执行生产设备管理制度;生产设备管理制度是否包括生产设备的采购、安装、确认、使用、维护保养、清洁等要求;
-
4.称量、配制、半成品贮存、填充与灌装、包装、产品检验等设备状态标识、清洁或者消毒标识是否清晰。
-
5.Are there written procedures for the cleaning and sanitation of equipment?是否有书面的设备清洁和卫生程序?-EN
-
6. Is the cleaning/sanitization procedure validated?清洁/卫生程序是否有效?-EN
-
7. Are there cleaning/sanitization records for the equipment?设备是否有清洁/卫生记录?-EN
-
8. Is the equipment cleaning/sanitization status labeled?设备清洁/卫生状态是否有标示?-EN
-
10. Is the equipment inspected for cleanliness before use and documented in the production record?设备在使用前是否进行清洁检查并记录在生产记录中? -EN
-
11.生产开始前,企业是否对生产车间环境、生产设备、周转容器状态和清洁(消毒)状态标识等进行确认,确保符合生产要求;
-
12. Is the equipment located to facilitate operations, cleaning and maintenance?设备的位置是否便于操作、清洁和维护?-EN
-
13. Is the equipment constructed of appropriate materials to prevent contamination and to facilitate cleaning?设备是否采用适当的材料来防止污染和便于清洗?-EN
-
14. Is the equipment designed to prevent contamination with lubricants and coolants?设备的设计是否能防止润滑油和冷却剂的污染?-EN
-
15. Are all major equipment identified and documented in the batch production record?批生产记录中是否有所有主要设备的标识和文件记录?-EN
-
16. Is the component (ingredient) addition verified by a second person?添加的组分(成分)是否由第二个人验证?-EN
-
企业是否建立并执行主要生产设备使用操作规程;操作规程、操作记录是否符合要求;
-
17. Are key processing steps and parameters documented in the production records i.e.. mixing speed, mixing times and temperatures?关键的加工步骤和参数是否记录在生产记录中,例如 搅拌速度,搅拌时间和温度?-EN
FILLING AND PACKAGING AREA
-
1.Whether the enterprise has established and implemented a production management system suitable for the variety, quantity and production license of cosmetics. At least include process and operation management, production instruction management, material requisitioning and inspection management, production environment management, production equipment management, production process management, production record management, material balance management, production clearance management, return material management, nonconforming product management, product release management and relevant traceability management systems;企业是否建立并执行与化妆品生产品种、数量和生产许可项目相适应的生产管理制度,至少包括工艺和操作管理、生产指令管理、物料领用和查验管理、生产环境管理、生产设备管理、生产过程管理、生产记录管理、物料平衡管理、生产清场管理、退仓物料管理、不合格品管理、产品放行管理以及有关追溯管理等方面的制度;
-
2.Whether the enterprise should improve the corresponding system dynamically according to the changes of the variety, quantity and production license items of cosmetics to ensure that they are valid versions in use.企业是否根据化妆品品种、数量和生产许可项目的变化动态完善相应制度,保证其在使用处为有效版本。
-
3.Whether the enterprise has formulated standardized production plans and issued production instructions according to the production plans;企业是否制定规范化的生产计划,是否依据生产计划下达生产指令;
-
4.Whether the production instructions include product name, production lot number (or the unique identifier associated with the production lot number), product formula, total production amount, production time, etc.生产指令是否包括产品名称、生产批号(或者与生产批号可关联的唯一标识符号)、产品配方、生产总量、生产时间等内容。
-
5.Whether the production instructions are effectively implemented in the actual production process;生产指令在实际生产过程中是否得到有效执行;
-
6.If stored for a period of time, is bulk retested for micro prior to use?如果贮存一段时间,在使用前是否对半成品微生物重新进行测试?-EN
-
7.Whether the enterprise has established and implemented the production process procedures and post operation procedures;企业是否建立并执行产品生产工艺规程和岗位操作规程;
-
8. . Whether the production process conforms to the technical requirements that have a substantial impact on product quality and safety;产品生产工艺规程是否符合对产品质量安全有实质性影响的技术性要求;
-
9.Whether the production process parameters and critical control points of the process are specified in the production process procedures of the enterprise.企业生产工艺规程中是否明确生产工艺参数及工艺过程的关键控制点。
-
10. Are there written filling and packaging control procedures approved by the QC unit?是否有书面的灌装和包装控制程序,经QC部门批准? -EN
-
11. Are product filling processes (manufacturing instructions / fill and assembly) validated by both factory and R&D?产品灌装工艺(生产说明书/灌装和装配指导书)是否经工厂和研发部门验证? -EN
-
12.Whether the enterprise has developed process verification management regulations; Whether the main production process has been verified; Whether the enterprise keeps verification schemes, records and reports;企业是否制定工艺验证管理规程;主要生产工艺是否经过验证;企业是否保存验证方案、记录及报告;
-
13.. When the main process parameters affecting product quality are changed, whether the enterprise will reverify.当影响产品质量的主要工艺参数等发生改变时,企业是否进行再验证。
-
14.Whether the enterprise has established and implemented a health management system for entering the production floor; Whether the health management system for entering the production plant includes cleaning, disinfection (if necessary), dress code, etc.; Whether the enterprise regularly cleans and disdisinfects the work clothes;企业是否建立并执行进入生产车间卫生管理制度;进入生产车间卫生管理制度是否包括进入生产车间人员的清洁、消毒(必要时)、着装要求等内容;企业是否定期对工作服清洁消毒;
-
15.. Whether the enterprise has established a management system for external personnel; Whether the alien personnel management system includes approval, registration, cleaning, disinfection (if necessary), clothing and safety guidance; Whether the enterprise supervises the external personnel;企业是否制定外来人员管理制度;外来人员管理制度是否包括批准、登记、清洁、消毒(必要时)、着装以及安全指导等内容;企业是否对外来人员进行监督;
-
16.Whether the enterprise carries out activities that have adverse effects on product quality and safety in the production workshop or laboratory, and whether it brings or places personal articles or other articles unrelated to production.企业是否在生产车间、实验室内开展对产品质量安全有不利影响的活动,是否带入或者放置与生产无关的个人用品或者其他与生产不相关物品。
-
17.Whether the enterprise has established and implemented the post-production cleaning and disinfection system;企业是否建立并执行生产后清洁消毒制度;
-
18.Whether the enterprise clears the site in time after production or before changing the production varieties, whether it cleans and disdisinfects the production area and production equipment, pipes and containers according to the prescribed methods and requirements, and whether it keeps records;企业在生产后或者更换生产品种前是否及时清场,是否按照规定的方法和要求对生产区域和生产设备、管道、容器具等清洁消毒,是否保留记录;
-
19.After the completion of cleaning and disinfection, whether the enterprise should clearly mark the effective period of cleaning and disinfection according to the regulations.清洁消毒完成后,企业是否按规定清晰标示清洁消毒有效期限。
-
20. Is line clearance between batches documented?是否有批次间的清线记录?-EN
-
21.Whether the cleaning and disinfection of the inner packing materials and its records meet the requirements of the corresponding operating procedures;内包材清洁消毒及其记录是否符合相应操作规程要求;
-
22.For clean packaging materials that do not require cleaning and disinfection, spot check whether they have a health compliance confirmation record.对无需清洁消毒的清洁包装材料,抽查是否具有卫生符合性确认记录。
-
23. Whether the lubricants, cleaners and disinfectants used by the enterprise cause pollution or corrosion to materials, products or equipment and appliances.企业所使用的润滑剂、清洁剂、消毒剂是否对物料、产品或者设备、器具造成污染或者腐蚀。
-
24.Whether the lubricants, cleaners and disinfectants used by the enterprise cause pollution or corrosion to materials, products or equipment and appliances.企业所使用的润滑剂、清洁剂、消毒剂是否对物料、产品或者设备、器具造成污染或者腐蚀。
-
25. Is the equipment inspected for cleanliness before use?设备在使用前检查是否干净?-EN
-
26. Is the compressed air that comes into product contact or contact with primary containers or other product contact surfaces oil-free and micro filtered?产品接触的压缩空气或与主要容器或其他产品接触表面接触的压缩空气是否无油和微过滤?-EN
-
27.Whether the labels of materials and semi-finished products used on the production site include name or code, production date or batch number, service life, quantity and other information;生产现场使用物料及半成品的标识是否包括名称或者代码、生产日期或者批号、使用期限、数量等信息;
-
28.Whether there is a record of material handover between various processes in the production process and whether it can be traced.生产过程中各工序之间物料交接是否有记录,是否可追溯。
-
29. Are actual yield calculations determined at the end of filling and verified by a second person?实际的产量计算是否在灌装结束时确定,并由第二个人验证?
-
30.Whether the enterprise has established and effectively implemented the post-production material balance management system;企业是否建立并有效执行生产后物料平衡管理制度;
-
31. Whether the material balance results of preparation, filling, filling, packaging and other processes meet the limits set by the production process regulations;配制、填充、灌装、包装等工序的物料平衡结果是否符合生产工艺规程设定的限度范围;
-
32.When the material balance deviates after production and exceeds the limit, whether the enterprise analyzes the reasons, whether the quality management department confirms that there is no potential quality risk before entering the next process, and whether the treatment process is recorded.生产后物料平衡出现偏差,超出限度范围时,企业是否分析原因,是否由质量管理部门确认无潜在质量风险后进入下一工序,是否记录处理过程。
-
33. Are labels and other proprietary components cleared, stored, and/or destructed post production?标签和其他专有组件是否在生产后被清除、存储和/或销毁?-EN
-
34. Is finished product examined during finishing to assure correct labeling? 成品在加工过程中是否经过检验以确保标签正确?-EN
-
35.企业是否建立并执行留样管理制度;留样管理制度是否明确产品留样程序、留存地点、留样数量、留样记录、保存期限和处理方法等内容;
-
36. Are production samples retained for at least four years from manufacture?生产样品是否从生产开始至少保留四年? -EN
-
37.Whether the enterprise retains samples for semi-finished products and finished products by batch; Whether the quantity of retained samples conforms to the regulations;企业是否对出厂的半成品、成品逐批留样;留样数量是否符合规定;
-
38.. If the products are finished products, whether the packaging of the retained samples conforms to the regulations;出厂的产品为成品的,留样的包装是否符合规定;
-
39.If the products are semi-finished products, whether the retained samples are sealed and the quality of the products is stable; Whether the label information includes the product name, enterprise name, specifications, storage conditions, service life and other information to ensure traceability.出厂的产品为半成品的,留样是否密封并保证产品质量稳定;标签信息是否包括产品名称、企业名称、规格、贮存条件、使用期限等信息,保证可追溯。
-
40.Whether the enterprise has set up a special sample retention area; Whether the storage conditions of the retained samples conform to the provisions of relevant laws and regulations and the requirements of labels;企业是否设置专门的留样区域;留样的贮存条件是否符合相关法律法规的规定和标签标示的要求;
-
41. Are finished product standards stored in an environmentally controlled area to prevent product contamination and degradation?成品标样是否存放在环境控制区域以防止产品污染和挥发?-EN
-
42. Whether the enterprise keeps the records of retained samples according to the regulations, and whether it records the quality of retained samples within the service life; Whether the retention period of the sample is no less than 6 months after the expiration of the product service life;企业是否按规定保存留样记录,是否记录留样在使用期限内的质量情况;留样的保存期限是否不少于产品使用期限届满后6个月;
-
43.Whether the enterprise conducts regular observation of the retained samples according to the retained samples management system; If it is found that the products with retained samples deteriorate within the service life, whether the enterprise should analyze the reasons in time, recall the batches of cosmetics already on the market according to law, and proactively eliminate safety risks.企业是否依据留样管理制度对留样进行定期观察;发现留样的产品在使用期限内变质时,企业是否及时分析原因,并依法召回已上市销售的该批次化妆品,主动消除安全风险。
-
44. Are finished product standards refreshed every 6 months?成品标样是否每6个月更新一次?
HOLDING AND DISTRIBUTION
-
1. Are there written warehousing procedures describing quarantine of manufactured products (bulk or finished goods) until approved by QC?是否有书面的仓储程序描述制成品(散装或成品)的检疫直至QC批准?-EN
-
2. Is there the SOP for the FG release?企业是否建立并执行产品放行管理制度;
-
3. Is rejected finished products status labeled or held in a quarantine area?被拒绝的成品是否有标签或保存在隔离区?-EN
-
4.Whether the enterprise has established and implemented the nonconforming product management system and rework control documents;企业是否建立并执行不合格产品管理制度和返工控制文件;
-
5. Are there written procedures for disposal of rejected finished product in a manner that assures it is not used? 是否有书面的程序处理不合格的成品,以确保它没有被使用?-EN
-
6. Is there a SOP that describes the requirements for product release, specification compliance, samples and documents?是否有一个SOP来描述产品放行、规范符合性、样品和文件的要求?-EN
-
7. Is there a SOP or system to limit the storage of manufactured bulk for a validated period of time?是否有一个SOP或系统来限制生产的半成品在有效的时间内的储存?-EN
-
8.Does company disposal the unqualified semi-FG in time and keep its record?企业是否按照不合格品管理制度及时处理超过使用期限未填充或者灌装的半成品,是否留存相关记录。
-
9. Are procedures in place for the proper notification and destruction of unacceptable/obsolete finished goods and/or package components?是否有适当的程序通知和销毁不可接受/废弃的成品和/或包装部件?
-
10. Do the company check and find the unqualified products in time?企业是否定期检查并且及时处理变质或者超过使用期限等质量异常的产品。
-
11. Is there the analysis report or record for the unqualified products? Do the QA department validation the unqualified products rework , disposal and keep the record?企业是否保存不合格品分析记录和分析报告;不合格产品的返工是否由质量管理部门按照返工控制文件予以评估确认;不合格品销毁、返工等处理措施是否由质量管理部门批准并记录。
LOSS PREVENTION(EN)
-
1. Are there adequate controls to prevent the loss of finished goods and/or componentry from the facility?工厂是否有足够的控制措施来防止成品和/或零部件的丢失?
-
2. Is facility access controlled and monitored after hours? Are the access records reviewed?下班后是否对设施的进出进行控制和监控? 是否审核了访问记录?
-
3. Are products shipped in a controlled & secured manner to prevent tampering, contamination or loss during transit?产品是否以受控和安全的方式运输,以防止在运输过程中篡改、污染或损失?
BUILDING AND FACILITIES
-
1.Whether the enterprise has production sites, facilities and equipment suitable for the variety, quantity and production license items of cosmetics produced企业是否具备与生产的化妆品品种、数量和生产许可项目等相适应的生产场地和设施设备;
-
Is the building maintained in a good state of repair?建筑物是否处于良好的维修状态?-EN
-
2.Whether there are dust, harmful gases, radioactive substances, garbage disposal and other diffuse pollution sources and toxic and harmful sites around the production site; Whether the building structure, production workshop and facilities of the enterprise are easy to clean, operate and maintain.生产场地周边是否有粉尘、有害气体、放射性物质、垃圾处理等扩散性污染源及有毒、有害场所;企业的建筑结构、生产车间和设施设备是否便于清洁、操作和维护。
-
3. The outside of the building is maintained free of trash and debris that provide harborage.建筑的外部没有垃圾和碎片。-EN
-
4. The building size and construction facilitate cleaning maintenance and operations.建筑物的大小和结构便于清洁、维护和操作。-EN
-
5. There is adequate space to prevent mix-ups of component (ingredient)s, labels, materials, and to prevent contamination.有足够的空间以防止组件(成分)、标签、材料的混淆,并防止污染。-EN
-
6.Whether the production workshop and other places store materials, products or other articles that have adverse effects on the quality and safety of cosmetics;生产车间等场所是否贮存对化妆品质量安全有不利影响的物料、产品或者其他物品;
-
7.If non-cosmetics are produced in the same production workshop, whether cosmetics prohibited raw materials and other raw materials that have adverse effects on the quality and safety of cosmetics are used, and corresponding measures are taken to prevent contamination and cross-contamination; Whether the enterprise has a risk analysis report to ensure that it does not have adverse impact on the quality and safety of cosmetics.共用生产车间生产非化妆品的,是否使用化妆品禁用原料及其他对化妆品质量安全有不利影响的原料,并具有防止污染和交叉污染的相应措施;企业是否有风险分析报告,确保其不对化妆品质量安全产生不利影响。
-
8. Doors and windows are kept closed.门窗都是关着的。-EN
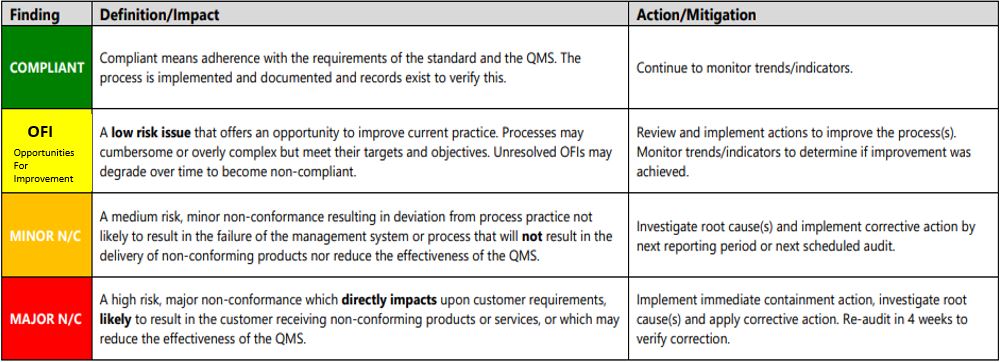
- Compliant
- OFI
- Minor N/C
- Major N/C
- N/A
-
9. Whether the enterprise sets up the production workshop according to the production process and environmental control requirements, and whether it arbitrarily changes the division of functional areas such as dressing, buffering, weighing, preparation, storage of semi-finished products, filling and filling, storage, packaging and storage of clean containers and instruments; (Is it consistent with the drawing?)企业是否按照生产工艺流程及环境控制要求设置生产车间,是否擅自改变更衣、缓冲、称量、配制、半成品贮存、填充与灌装、清洁容器与器具贮存、包装、贮存等功能区域划分;(与图纸是否一致)
-
10. Whether there are pollution sources in the production workshop; Whether the flow of materials, products and personnel is reasonable, and whether there are any circumstances leading to material and product contamination and cross contamination.生产车间内是否有污染源;物料、产品和人员流向是否合理,是否存在导致物料、产品污染和交叉污染的情形。
-
11.Whether the dressing room of the production workshop is equipped with wardrobe and shoe cabinet; Clean areas and quasi-clean areas are equipped with non-hand contact hand washing and disinfection facilities commensurate with the number of personnel;生产车间更衣室是否配备衣柜、鞋柜;洁净区、准洁净区是否配备与人员数量相匹配的非手接触式洗手及消毒设施;
-
12.Whether the enterprise should set up secondary changing rooms according to the requirements of production environment control.企业是否根据生产环境控制需要设置二次更衣室。
-
13.Whether the enterprise divides the production area according to the requirements of product process environment; Whether the environmental indicators of the production workshop meet the requirements of Appendix 2 of the Cosmetic production quality Management Code;企业是否按照产品工艺环境要求划分生产区域;生产车间环境指标是否符合化妆品生产质量管理规范附2的要求(半成品贮存①、填充、灌装,清洁容器与器具贮存、称量、配制、缓冲、更衣,空气中细菌菌落总数③: ≤1000cfu/m3)(①企业配制、半成品贮存、填充、灌装等生产工序采用全封闭管道的,可以不设置半成品贮存间,③测试方法参照《GB 15979 一次性使用卫生用品卫生标准》或者《GB/T 16293 医药工业洁净室(区)浮游菌的测试方法》的有关规定。)
-
14.Whether the areas of different cleanliness levels are physically isolated and whether the corresponding pressure difference is maintained according to the process quality assurance requirements.不同洁净级别的区域是否物理隔离,是否根据工艺质量保证要求,保持相应的压差。
-
15. Operations are performed within specifically defined areas to prevent contamination or mix-ups.操作必须在特定的区域内进行,以防止污染或混淆。-EN
-
16.For the production process that is easy to produce dust and not easy to clean (powder, nail polish, perfume and other products), whether to set up a separate production operation area and whether to use special production equipment;易产生粉尘、不易清洁等(散粉类、指甲油、香水等产品)的生产工序,是否设置单独生产操作区域,是否使用专用生产设备;
-
17.For the production process of hair dye, perm, wax base and other products that are not easy to clean, whether to set up a separate production operation area or physical partition, and whether to use special production equipment;染发类、烫发类、蜡基类等产品不易清洁的生产工序,是否设置单独生产操作区域或者物理隔断,是否使用专用生产设备;
-
18.Whether the production process which is easy to produce dust and not easy to clean should take corresponding cleaning measures to prevent cross pollution;易产生粉尘、不易清洁等的生产工序是否采取相应的清洁措施,防止交叉污染;
-
19.Whether the operation area of the production processes prone to dust generation and the use of volatile substances (e.g. weighing, screening, crushing, mixing, etc.) is equipped with effective dust removal or exhaust facilities.易产生粉尘和使用挥发性物质的生产工序(如称量、筛选、粉碎、混合等)的操作区是否配备有效的除尘或者排风设施。
-
20. Is there adequate lighting?是否有足够的照明?-EN
-
21.Whether the workshop is equipped with facilities to prevent mosquitoes, flies, insects, rats and other animals from entering and breeding, whether it is effectively monitored and recorded, and whether the risks are regularly analyzed;生产车间是否配备防止蚊蝇、昆虫、鼠和其他动物进入、孳生的设施,是否有效监控并留存记录,是否定期分析存在的风险;
-
The building is free of signs of infestation.这座建筑没有虫害的迹象。-EN
-
There are written procedures for pest control including a drawing or floor plan showing the placement of control devices.有书面的虫害控制程序,包括显示控制装置放置位置的图纸或平面图。-EN
-
22.Materials, products and other storage areas are equipped with appropriate lighting, ventilation, rodent, pest, dust, moisture and other facilities; Whether the enterprise has established the relevant management system, set the temperature and humidity range, and equipped the temperature and humidity adjustment and monitoring facilities according to the characteristics of materials and products.物料、产品等贮存区域是否配备合适的照明、通风、防鼠、防虫、防尘、防潮等设施;企业是否制定相关管理制度,设置温度、湿度范围,是否依照物料和产品的特性配备温度、湿度调节及监控设施。
-
23. Whether the design, installation, operation and maintenance of the enterprise air purification system can ensure that the production workshop meets the environmental requirements; Whether the company keeps the drawings related to the design and installation of the air purification system and the operation and maintenance records.企业空气净化系统的设计、安装、运行、维护是否可以确保生产车间达到环境要求;企业是否保留空气净化系统设计、安装相关图纸及运行、维护记录。
-
24.Whether the enterprise has established and implemented the system of regular cleaning, disinfection, monitoring and maintenance of the air purification system, whether it has implemented corresponding measures according to the system, and should keep relevant records.企业是否建立并执行空气净化系统定期清洁、消毒、监测、维护制度,是否按照制度落实相应措施,并应留存相关记录。
-
25. Is there adequate filtered ventilation to production operations where product is exposed?产品暴露的生产操作是否有足够的过滤通风?-EN
-
26.Whether the production workshop maintains good ventilation and appropriate temperature and humidity; Whether the temperature and humidity are within the specified range;生产车间是否保持良好的通风和适宜的温度、湿度;温度、湿度是否在规定的区间范围内;
-
27.Whether the enterprise has established the clean area purification and disinfection and quasi-clean area disinfection management system according to the production process needs, to ensure the effective implementation of relevant measures, and whether the system is implemented and recorded;企业是否根据生产工艺需要,制定洁净区净化和消毒、准洁净区消毒管理制度,确保相关措施的有效实施,是否按制度执行并记录;
-
28.Whether the enterprise has developed environmental monitoring plans for clean areas and quasi-clean areas, and whether it regularly monitors and records the plans; According to the environmental monitoring plan, the company shall inspect the cosmetics production workshop every year in accordance with the environmental requirements of the cosmetics production workshop.企业是否制定洁净区和准洁净区环境监控计划,是否按照计划定期监控并记录;企业是否每年根据环境监控计划,按照化妆品生产车间环境要求对生产车间进行检测。
-
29. There are adequate drains for equipment and to prevent standing water.有足够的排水设备和防止存水。-EN
-
30, Whether the enterprise has established and implemented the operation procedures for cleaning or disinfecting production equipment, pipes, containers and utensils; Whether the cleaning or disinfection operation procedures include cleaning and disinfection methods, names and preparation methods of cleaning agents and disinfectants, requirements on cleaning water and cleaning utensils, and cleaning validity period, etc.; Whether the enterprise has made clear the basis for the selection of cleaning or disinfection methods;企业是否建立并执行生产设备、管道、容器、器具的清洁或者消毒操作规程;清洁或者消毒操作规程是否包括清洁消毒方法、清洁剂和消毒剂的名称与配制方法、清洁用水和清洁用具要求、清洁有效期限等内容;企业是否明确清洁或者消毒方法选择的依据;
-
31. Are there written procedures for the cleaning and sanitization of filling equipment?灌装设备是否有书面的清洗和消毒程序?-EN
-
32,Are cleaning and sanitization methods validated to prevent chemical residue on equipment?清洗和消毒方法是否有效,以防止化学品残留在设备上? -EN
-
33,Are the cleaning and sanitization procedures for filling equipment validated?灌装设备的清洗和卫生程序是否有效?-EN
-
34,Are there cleaning/sanitation records for the filling equipment?灌装设备是否有清洁/卫生记录?-EN
-
35.Whether the enterprise is equipped with equipment suitable for the variety, quantity, production license items and production process of cosmetics produced;企业是否配备与生产的化妆品品种、数量、生产许可项目、生产工艺流程相适应的设备;
-
36.Whether the weighing, preparation, semi-finished product storage, filling and filling, packaging, product inspection and other equipment related to product quality and safety are set with unique numbers;与产品质量安全相关的称量、配制、半成品贮存、填充与灌装、包装、产品检验等设备是否设置唯一编号;
-
37,Is the equipment located to facilitate operations, cleaning and maintenance?设备的位置是否便于操作、清洁和维护?-EN
-
38,Is the equipment constructed of appropriate materials to prevent contamination and to facilitate cleaning? 设备是否采用适当的材料来防止污染和便于清洗?-EN
-
39.Whether the design and installation of pipelines avoid dead corners, blind pipes or contamination; Whether the name of the contents or the purpose of the pipe is clearly marked on the fixed pipe, and whether the direction of flow is indicated.管道的设计、安装是否避免死角、盲管或者受到污染;固定管道上是否清晰标示内容物的名称或者管道用途,是否注明流向。
-
40,Is the equipment designed to prevent contamination with lubricants and coolants? 设备的设计是否能防止润滑油和冷却剂的污染?-EN
-
41.Whether the materials of all the equipment, appliances and pipes in contact with raw materials, inner packing materials and products meet the usage requirements and whether the quality and safety of products are affected.所有与原料、内包材、产品接触的设备、器具、管道等的材质是否满足使用要求,是否影响产品质量安全。
Record & Documentation Review
BATCH PRODUCTION RECORDS
-
1.Whether the enterprise has established a document management system; Whether the document management system specifies the procedures and formats for the formulation, examination, approval, issuance, invalidation and destruction of documents of the quality management system;企业是否建立文件管理制度;文件管理制度是否明确质量管理体系文件制定、审核、批准、发放、作废、销毁等的程序和格式;
-
2.Whether the enterprise implements the document management system; Whether the documents are controlled, whether they have been examined and approved, whether the versions stored in the use place are valid, whether the external documents are updated in time, whether the invalid documents are destroyed in time, etc.企业是否执行文件管理制度;文件是否受控、是否经审核批准、在使用处存放的是否为有效版本,外来文件是否及时更新,作废文件是否及时销毁等。
-
3.Whether the enterprise has established a record management system; Whether the record management system specifies the procedures and formats of record filling, preservation and disposal;企业是否建立记录管理制度;记录管理制度是否明确记录的填写、保存、处置等程序和格式;
-
4.Whether the enterprise implements the record management system and fills the records in time; Whether the records are true, complete, accurate, clear and easy to distinguish, interrelated and traceable; Whether there are arbitrary changes in the records; Whether the corrections to the records meet the requirements.企业是否执行记录管理制度,是否及时填写记录;记录是否真实、完整、准确,清晰易辨,相互关联可追溯;记录是否存在随意更改的情况;记录的更正是否符合要求。
-
5.If the enterprise uses computer (electronic) system to generate and save records or data, whether it meets the requirements of Appendix 1 of the Cosmetic production quality Management Code. It mainly includes:1.) Whether the system using electronic records meets the specified functional requirements;2.) Whether the effectiveness and security of the system have been verified;3. )Whether the system has effective measures to ensure data security, such as regular backup to prevent viruses and illegal intrusion;4.) Whether the system can ensure the uniqueness and traceability of login users;5.) Whether electronic records can achieve the same function as paper records; Whether the data or information generated and saved by the system is true, complete, accurate and traceable;6. )Whether the system has established an effective trajectory automatic tracking system, which can automatically track the operation of login, editing, modification, deletion, system setting, calibration, modification, time stamp change and so on, and trace the operator, operation time and operation process.企业采用计算机(电子化)系统生成、保存记录或者数据的,是否符合化妆品生产质量管理规范附1的要求。主要包括:1.采用电子记录的系统是否满足规定的功能要求;2.系统的有效性和安全性是否经过验证;3.系统是否具有保证数据安全性的有效措施,例如定期备份,防止病毒和非法入侵等;<br>4.系统是否可以确保登录用户的唯一性与可追溯性;<br>5.电子记录能否实现与纸质记录同等功能;系统生成和保存的数据或者信息是否真实、完整、准确、可追溯;<br>6.系统是否建立有效的轨迹自动跟踪系统,能够对登录、编辑、修改、删除以及系统的设置、校准、修改、时间戳变更等操作进行自动跟踪,追溯操作者、操作时间和操作过程。
-
6.Whether the enterprise has a record of the activities related to the cosmetics production quality management practices; Whether it includes personnel training, health and hygiene management, environmental monitoring, facilities, equipment, instruments cleaning, disinfection, monitoring, use, maintenance management, supplier review and evaluation, material purchase, acceptance, storage, use management, product production, release management, non-conforming product management, inspection management, sample management, laboratory management, system self-inspection, Sales, returns, complaints, recalls, adverse reaction monitoring and other activity records企业是否对与化妆品生产质量管理规范有关的活动均形成了记录;是否包括人员培训、健康、卫生管理,环境监控,设施、设备、仪器的清洁、消毒、监测、使用、维护管理,供应商审核评价,物料采购、验收、贮存、使用等管理,产品生产、放行管理,不合格品管理,检验管理,留样管理,实验室管理,体系自查,销售、退货、投诉、召回、不良反应监测等活动记录。
-
7.Whether the enterprise has established and implemented the traceability management system; Whether to specify the definition of batch and the batch number management rules of raw materials, inner packing materials, semi-finished products and finished products;企业是否建立并执行追溯管理制度;是否明确规定批的定义以及原料、内包材、半成品、成品的批号管理规则;
-
8.Can the enterprise ensure that all records related to the production of each batch of products are related to each other through batch number management;企业能否通过批号管理确保与每批产品生产相关的所有记录相互关联;
-
9.Can the company ensure that all activities such as material purchase, product production, quality control, storage, sales and recall can be traced?企业能否保证物料采购、产品生产、质量控制、贮存、销售和召回等全部活动可追溯。
-
10.Whether the production records can truthfully reflect the technical parameters and key point control status of the entire production process, and whether they include production instructions, material acquisition, weighing, preparation, filling or filling, packaging process, product inspection and release records, etc.生产记录是否可以如实反映出整个生产过程的技术参数和关键点控制状况,是否包括生产指令、领料、称量、配制、填充或者灌装、包装过程和产品检验、放行记录等内容。
-
11. Is the QC unit review of the records documented?QC部门对记录的审核是否有文件记录?-EN
-
12. Does the review result in a shipping release document?审核结果是否形成出货放行文件?-EN
-
13. Was all testing completed before the shipping release was signed? 所有的测试是否在发货放行单签署前完成?-EN
-
14. Is the Formula change control documented?配方变更控制是否有文件记录?-EN
-
15. For first production has the contract manufacturer established an internal procedure to identify the First Production batches. Give written notification at least 48 hours before start-up. Assure that before start-up there is an approved formula, approved standard, approved specification, fill and assembly specification and packaging specification?对于第一次生产,合同制造商建立了一个内部程序来识别第一次生产批次。 至少在启动前48小时书面通知。 确保在启动前有一个批准的配方,批准的标准,批准的规格,填充和组装规格和包装规格?-EN
-
16.Whether all records are clearly marked and stored in an orderly manner for easy access;所有记录是否标示清晰,存放有序,便于查阅;
-
17. Are all batch related instructions and forms combined into a comprehensive batch packet? Are records retrievable within 24 hours?是否将所有与批处理相关的指令和表格合并成一个完整的批记录包? 记录能在24小时内恢复吗?-EN
-
18. Are batch records for retained for at least 4 years and/or the time period required by local regulations? 批记录是否至少保留4年和/或当地法规要求的时间期限?-EN
REGISTERED FACILITY
-
1. Whether the enterprise has established an organizational structure, and whether the organizational structure is compatible with the variety, quantity and production license of cosmetics produced;企业是否建立组织机构,组织机构是否与生产的化妆品品种、数量和生产许可项目相适应;
-
2. Whether the enterprise has made written provisions on the responsibilities and authority of the quality management and production departments;企业是否对质量管理、生产等部门职责权限做出书面规定;
-
3.Whether the enterprise is equipped with managers, operators and inspectors suitable for the variety, quantity and production license items of cosmetics it produces; Whether the personnel assigned meet the corresponding job conditions.企业是否配备与其生产的化妆品品种、数量和生产许可项目等相适应的管理人员、操作人员和检验人员;配备的人员是否满足相应的任职条件。
-
4. Has the facility been registered with local governing agencies as a cosmetic, chemical, or similar manufacturing facility and has received approval to manufacture legally within the guidelines as specified by the registration? 工厂是否已在当地管理机构注册为化妆品、化学品或类似的生产工厂,并已获得批准在注册规定的范围内合法生产?-EN
-
5. Has the facility been certified, under local governing agencies and/or to a international standard for the manufacturing of cosmetic, chemical or similar products? (Ex. IS0 9001, ISO 22716 etc.) 工厂是否通过了当地管理机构和/或化妆品、化学品或类似产品制造的国际标准的认证? (如IS0 9001、ISO 22716等)-EN
-
6. Whether the products produced by the enterprise meet the relevant laws and regulations, compulsory national standards, technical specifications and technical requirements set forth in the registration and filing materials of cosmetics.企业生产的产品是否符合相关法律法规、强制性国家标准、技术规范和化妆品注册、备案资料载明的技术要求。
-
7. When was the last local government inspection? Have any major non-compliances and/or fines been issued in the past 10 years?上次当地政府检查是什么时候? 在过去10年是否有重大违规及/或罚款?-EN
-
8. Are batch production records retained for one year past expiration date?批生产记录是否在产品过期后保留一年? -EN
-
9. Are there written procedures for an annual review of written records; batch production records, complaints, recalls and investigations?是否有对书面记录进行年度评审的书面程序(批生产记录,投诉,召回和调查记录)?-EN
-
10. Is there a record of the most recent required review for Mast products? Or an example of a review from a similar customer? 是否有Mast产品最近要求的审核记录? 或者一个类似客户的记录审核的例子?-EN
-
11.. Whether the enterprise carries out self-check on the implementation of cosmetic production quality management standards every year; If it is found that the production conditions do not meet the requirements of the cosmetic production quality management code, whether to take corrective measures immediately; Whether to stop production immediately and report to the drug regulatory department of the province, autonomous region or municipality directly under the Central Government where the quality and safety of cosmetics may be affected;企业是否每年对化妆品生产质量管理规范的执行情况进行自查;在发现生产条件不符合化妆品生产质量管理规范要求时,是否立即采取整改措施;在发现可能影响化妆品质量安全时,是否立即停止生产并向所在地省、自治区、直辖市药品监督管理部门报告;
-
12. If the enterprise has stopped production for more than 1 year continuously, whether it should carry out a comprehensive self-inspection according to regulations before resuming production to confirm that it meets the requirements of cosmetic production quality management standards; Whether the situation of self-inspection and rectification should be reported to the drug regulatory department of the local province, autonomous region or municipality directly under the Central Government within 10 working days from the date of resumption of production; If it is found that the production conditions do not meet the requirements of the cosmetic production quality management code, whether to take corrective measures immediately; Whether to stop production immediately and report to the drug regulatory department of the province, autonomous region or municipality directly under the Central Government where the quality and safety of cosmetics may be affected;企业有连续停产1年以上的情形时,是否在重新生产前按规定开展全面自查,确认符合化妆品生产质量管理规范要求后再恢复生产;自查和整改情况是否在恢复生产之日起10个工作日内向所在地省、自治区、直辖市药品监督管理部门报告;
-
13. in case of unqualified cosmetic sampling inspection results, whether the enterprise shall conduct self-inspection and rectification in time according to regulations.企业在出现化妆品抽样检验结果不合格时,是否按照规定及时开展自查并进行整改。
QUALITY CONTROL
-
1.Does the company QMS include below process documents: Quality Manual, Quality goal, Quality management system, quality standard, product formular, manufacturing process SOP, regulation and others?企业建立的化妆品生产质量管理体系文件是否健全,是否包括质量方针、质量目标、质量管理制度、质量标准、产品配方、生产工艺规程、操作规程,以及法律法规要求的其他文件;
-
2.Does the quality policy show the quality target and informed all the employees in the company? Is there any data in the quality target? Does the QMS reasonable and can be followed? Is there any product materials or QA requirement mentioned in the QA standard? Does the notification document same as FLF? Is there any SOP covered the key position or key equipment? 企业是否制定能体现质量方向的质量方针,并向全员宣贯;质量目标是否有量化指标;质量管理制度是否适宜并可操作;质量标准是否涵盖物料和产品的质量要求;操作规程是否涵盖关键岗位和关键仪器设备操作要求。
-
5.Does the company have the cosmetic quality and safety responsibility? Are there any written notice identify the key position responsibility for company representative, quality and safety responsible person, quality leader, manufacturing leader and other key possibility?企业是否建立化妆品质量安全责任制;是否书面规定企业法定代表人、质量安全负责人、质量管理部门负责人、生产部门负责人以及其他化妆品质量安全相关岗位的职责;
-
6.Are the key position person full fill their responsibility?企业各岗位人员是否按照其岗位职责的要求逐级履行质量安全责任。
-
7 .Is there a written noticed the REP take the integrity responsibility for the cometic quality and safety?企业是否书面明确规定法定代表人全面负责化妆品质量安全工作;
-
8.Does the company REP provide appropriate resource according product category, QTY and manufacturing items? Does the company REP lead to identify the quality principle and target ? Also lead the audit and analysis?法定代表人是否为化妆品生产和质量安全工作提供与生产化妆品品种、数量和生产许可项目相适应的资源,是否组织制定企业的质量方针和质量目标,是否组织对质量目标的实现进行定期考核和分析。
-
9.Does the quality and safety responsibility person performed the duties independently for quality /safety management and product released without outside disturbing.质量安全负责人是否按照质量安全责任制独立履行职责,在产品质量安全管理和产品放行中不受企业其他人员的干扰;
-
10. Is there a process document clarify the quality and safety responsibility person appointing other persons perform the duties with the REP agreement in the written version? 质量安全负责人指定本企业的其他人员协助履行其职责的,指定协助履行的职责是否为化妆品生产质量管理规范第七条第二款(一)(二)项以外的职责;是否制定相应的指定协助履行职责管理程序并经法定代表人书面同意;
-
11.Does the quality and safety responsibility person lead the QMS and clarify the related responsibility and report to the REP by the written method?质量安全负责人是否建立并组织实施本企业质量管理体系,落实质量安全管理责任,并定期以书面报告形式向法定代表人报告质量管理体系运行情况;
-
12. Is the quality and safety responsibility person in charge of the decision and the key documents announce for quality and safety parts.质量安全负责人是否负责产品质量安全问题的决策及有关文件的签发;
-
13.Does the quality and safety responsibility person identify the other supporting persons’ duties? Do the other supporting persons perform the duties well including all the record..质量安全负责人指定本企业的其他人员协助履行其职责的,被指定人员在协助履职过程中是否执行相应的管理程序,并如实记录,保证履职的内容、时间、具体事项可追溯;
-
15. Does the quality and safety responsibility person supervise the supporting situation?质量安全负责人是否对协助履职情况进行监督。
-
16.Does the company have the independent quality department ?企业是否独立设置质量管理部门且配备相应办公场所及专职人员;
-
17.Are there JD or authority clarified for the management department ?企业是否明确质量管理部门岗位职责和权限,并规定参与质量管理活动的内容;
-
18.Does the quality department perform their duties well?质量管理部门是否按照其职责范围履行质量管理职责。
-
19.Does the quality department leader audit all the QA documents (including SOP, process, record ,report and so on)?质量管理部门负责人是否承担所有产品质量有关文件(包括制度、程序、标准、记录、报告等)的审核管理;
-
20.Does the quality department leader lead the activity including the change management, internal audit ,unqualified product management, product recall ,etc?质量管理部门负责人是否根据质量管理体系要求,组织与产品质量相关的变更、自查、不合格品管理、召回等活动;
-
21.Does the quality leader supervise the QA standard,validated method and other quality control parts?质量管理部门负责人是否监督保证质量标准、检验方法和其他质量管理规程有效实施;
-
22.Does the QA leader validate or check the manufacturing process( including parameters, and key control points) and related report?质量管理部门负责人是否组织实施主要生产工艺(包括生产工艺参数、工艺过程的关键控制点)等必要的验证工作,并审核和批准验证方案和报告;
-
25. Is the QA leader in charge of other items which linked with product quality?质量管理部门负责人是否负责其他与产品质量有关的活动;
-
26.Does the manufacturing leader validate the production SOP according the production notification information to make sure they are same?生产部门负责人是否根据相应的生产管理规程,保证产品按照化妆品注册、备案资料载明的技术要求以及企业制定的生产工艺规程和岗位操作规程生产;
-
27.Does the manufacturing leader make sure all the production record real, integrity ,correct and could be tracked?生产部门负责人是否根据相应的生产管理规程,保证生产记录真实、完整、准确、可追溯;
-
28.生产部门负责人是否根据相应的生产管理规程,保证生产环境、设施设备满足生产质量需要;Is the manufacturing leader in charge of all production activities and make sure they are match the quality requirement including the production environment, equipment and others.
-
29.Whether the head of the production department is responsible for other activities related to the production of products;生产部门负责人是否负责其他与产品生产有关的活动;
-
30. Do the quality safety responsibility person or quality leader also take the manufacturing leader?质量安全负责人、质量管理部门负责人是否兼任生产部门负责人。
-
34.For each validation items, does the company identify clearly for the method, sampling requirement, sample management, test SOP, validation process , result dealing etc.?企业是否对每种检验对象规定检验或者确认方法、取样要求、样品管理要求、检验操作规程、检验过程管理要求以及检验异常结果处理要求等;
-
35. Is the validation result are real, integrity and correct which should match the previous test?企业检验或者确认的结果是否真实、完整、准确;检验结果是否与检验原始记录保持一致。
-
39 .Is there the SOP for semi-FG life time and its reference?企业是否建立半成品使用期限管理制度;设定的半成品使用期限是否有依据;
-
42. Before FG release, will the products validate items matched the SPEC? Do the quality and safety responsibility person validate the production and quality record? 产品放行前,企业是否确保产品经检验合格且检验项目至少包括出厂检验项目;是否确保相关生产和质量活动记录经质量安全负责人审核批准。
-
43 .Do the company identify the FG SPEC clearly? 企业是否明确规定化妆品出厂检验项目。
-
44. Are finished goods inspected properly by QC prior to release for shipping?成品在发货前是否经QC检验?
-
45. Does the QC Unit review production records prior to release or shipping the product?QC部门是否在放行或发运产品前审核生产记录?-EN
-
47. Is there history of internal and/or external quality holds, withdrawals or recalls? 是否有内部和/或外部质量持有,撤回或召回的历史? -EN
-
48. Is batch data trended to ensure processes are in control?是否有批次数据,以确保所有流程的受控? -EN
-
49. Is there an appropriate CAPA system in place to identify and resolve quality based issues?是否有适当的CAPA系统来识别和解决质量问题?-EN
-
51 .Is there the check list and report for the internal audit?企业是否在实施质量管理体系自查前制定自查方案,是否在自查完成后形成自查报告;
-
52. Does the internal audit report include the findings, product quality safety evaluation, improvement items, etc. which should validated by the REP, quality and safety responsibility person and keep related departments informed>自查报告是否包括发现的问题、产品质量安全评价、整改措施等内容;自查报告是否经质量安全负责人批准,是否报告法定代表人,是否反馈企业相关部门;
-
53. Do the company track the CAPA?企业是否对整改情况进行跟踪评价。
-
55 Does the manufacturer mention on the label finish the last production step ?.内包材上标注标签的生产工序是否在完成最后一道接触化妆品内容物生产工序的生产企业内完成。
-
56. Does the warehouse record ,sales record same as the product?所销售产品的出货单据与产品实物是否一致。
-
57. Have the company changed the product life time?企业是否存在擅自更改产品使用期限的行为。
-
58. Do the products on the market have the COA or qualified proof?上市销售的产品是否附有出厂检验报告或者合格标记等形式的产品质量检验合格证明。
-
60. Is the customs compliance process including the record, investigation, evaluation, deal etc.?企业是否建立并执行产品质量投诉管理制度;产品质量投诉管理制度是否规定投诉登记、调查、评价和处理等要求;
-
61. Is the identified person in charge of the quality compliance and keep the record who has the knowledge to deal with the compliance?企业是否指定人员负责产品质量投诉处理并记录;指定的人员是否具备质量投诉处理的基本知识;
-
62. Do the QA department analysis the compliance and make the improvement action?企业质量管理部门是否对质量相关投诉内容进行分析评估,并采取措施提升产品质量。
PERSONNEL QUALIFICATIONS
-
1.Whether the enterprise has formulated the on-boarding training and annual training plans for employees; Whether the training plan reasonably sets legal knowledge, professional knowledge, operational skills and other contents according to the variety and quantity of cosmetics produced and production license items;企业是否制定从业人员入职培训和年度培训计划;培训计划是否根据生产的化妆品品种、数量和生产许可项目合理设置法律知识、专业知识以及操作技能等内容;
-
2. Whether the company conducts employee training in accordance with the on-boarding training and annual training plan; Whether the training effect has been assessed;企业是否按照入职培训和年度培训计划对员工进行培训;培训效果是否经过考核;
-
3.Whether the new employees or transferred employees can start their jobs after passing the assessment of job knowledge, job responsibilities and operational skills; Whether the employee has the corresponding ability to perform the job;新入职员工或调岗员工是否经岗位知识、岗位职责和操作技能考核合格后上岗;员工是否具备相应履职能力;
-
4.Whether the enterprise has established employee training files; Whether the training files include training personnel, time, content, method and assessment, etc.企业是否建立员工培训档案;培训档案是否包括培训人员、时间、内容、方式及考核情况等。
-
5. Is there a record of training in GMP and in the particular operations each employee performs?是否有每个员工在GMP和特定操作方面的培训记录? -EN
-
6. Is the training conducted by a qualified individual and is a retraining cadence specified?培训是否由有资质的人进行,是否规定了再培训的计划?-EN
-
7.Whether the enterprise has a person in charge of quality and safety;企业是否设有质量安全负责人;
-
8.Whether the quality and safety responsible person has professional education or training background in cosmetics, chemistry, chemical engineering, biology, medicine, pharmacy, food, public health or law, whether he has professional knowledge related to cosmetic quality and safety, and whether he is familiar with relevant laws and regulations, mandatory national standards and technical specifications;质量安全负责人是否具备化妆品、化学、化工、生物、医学、药学、食品、公共卫生或者法学等专业教育或培训背景,是否具备化妆品质量安全相关专业知识,是否熟悉相关法律法规、强制性国家标准、技术规范;培训计划和培训记录体现质量安全负责人年度培训学时不低于40小时。
-
9. Whether the person in charge of quality and safety has more than 5 years of experience in cosmetics production or quality management.质量安全负责人是否具有5年以上化妆品生产或者质量管理经验。
-
10. If the person in charge of quality and safety designates other personnel of the company to assist in performing their duties, whether the designated personnel has the corresponding qualification and ability to perform their duties;质量安全负责人指定本企业的其他人员协助履行其职责的,被指定人员是否具备相应的资质和履职能力;
-
11.Whether the enterprise has a person in charge of quality management department;企业是否设有质量管理部门负责人;
-
14. Whether the person in charge of the quality management department shall formulate the induction training and annual training plan related to production quality management according to the actual situation of the enterprise, and carry out the training and assessment according to the training plan to ensure that the staff can meet the requirements of the job responsibilities;质量管理部门负责人是否根据企业实际情况制定生产质量管理相关的入职培训和年度培训计划,并根据培训计划实施培训及考核,以保证员工达到岗位职责的要求;
-
15.Whether the enterprise has a person in charge of the production department;企业是否设有生产部门负责人;
-
16.Whether the person in charge of the production department has professional education or training background in cosmetics, chemistry, chemical engineering, biology, medicine, pharmacy, food, public health or law, whether he has professional knowledge related to cosmetic quality and safety, and whether he is familiar with relevant laws and regulations, mandatory national standards and technical specifications;生产部门负责人是否具备化妆品、化学、化工、生物、医学、药学、食品、公共卫生或者法学等专业教育或培训背景,是否具备化妆品质量安全相关专业知识,是否熟悉相关法律法规、强制性国家标准、技术规范;
-
17. Whether the head of the production department has experience in cosmetics production or quality management;生产部门负责人是否具有化妆品生产或者质量管理经验;
-
18.Whether the head of the production department confirms the training content of the employees directly engaged in production activities, defines the training effect, and ensures that they have the knowledge and skills appropriate to the post requirements;生产部门负责人是否确认直接从事生产活动的员工培训内容,明确培训效果,保证其具备与岗位要求相适应的知识和技能;
-
19. Whether the enterprise conducts production process training for production operators; Whether the operators operate in accordance with the technical parameters and key control requirements specified in the production process and post operation procedures;企业是否对生产操作人员进行生产工艺培训;操作人员是否按照生产工艺规程和岗位操作规程规定的技术参数和关键控制要求进行操作;
-
20. Do the personnel use protective apparel such as head, face and arm coverings properly to prevent product contamination?员工是否正确使用防护服,如头、脸和手臂覆盖物,以防止产品污染?-EN
-
21,Is there the SOP for the employees’ health management?企业是否建立并执行从业人员健康管理制度;
-
22,Do the cosmetic operators do the physical check before on board and keep it every year? Do they have any diseases which can’t follow the cosmetic work?直接从事化妆品生产活动的人员是否在上岗前接受健康检查,是否在上岗后每年接受健康检查;直接从事化妆品生产活动的人员是否患有国务院卫生主管部门规定的有碍化妆品质量安全疾病;
-
23,Do the company have the employees’ health record and keep it as long as the government requirement?企业是否建立从业人员健康档案;健康档案保存期限是否符合要求。
DEVELOPMENT TESTING(EN)
-
1. If applicable, are OTC products formulated to provide not less than 100% of the labeled amount of active ingredient?如果适用,OTC产品的配方是否提供不低于标签上有效成分含量的100% ?
-
2. Was preservative challenge testing completed on the product formulation prior to first production?在首次生产前是否完成了产品配方的防腐剂挑战测试?
-
3. Was RIPT testing completed before first production? (Human Repeat Insult Patch Test)RIPT测试在第一次生产前完成了吗? (人类重复斑贴测试)
-
4. Is there a written stability program? Does this stability program meet Mast stability testing protocols?是否有书面的稳定性计划? 这个稳定性程序是否符合Mast稳定性测试协议?
-
5. Is there documentation of the setting of the tentative expiration date?是否有关于暂定有效期的文件?
-
6.s the DOT hazardous material labeling assessment documented?交通部危险物质标签评估是否有文件记录?
-
7. Are there actual shelf life studies underway to support the tentative expiration date?是否有实际的保质期研究来支持暂定的保质期?
-
8. Is there preservative challenge testing on aged samples to show the preservative effectiveness at expiration?是否对陈年样品进行防腐剂挑战测试以显示过期的防腐剂有效性?
-
9. If applicable, is there a test method validation for the active ingredient assay?如果适用,是否有有效成分测定的测试方法验证?
SIGN-OFF
-
Auditor's Name & Signature审核员名字&签字: