Title Page
-
Site conducted
-
Facility工厂:
-
Audit Date & Time审核日期&时间:
-
Attendees参与者:
-
Auditor审核员:
-
Location地址:
-
Add location
Facility Review
RECEIVING
-
1. Are there written procedures for the receipt, identification, storage, handling, sampling, testing and approval of raw materials and labels?对原材料和标签的接收,标识,储存,加工,取样,测试和批准是否有相关的纸质程序文件?
-
2. Are raw materials withheld from production until released by Q.C.?原材料是否在QC放行前不用于生产?
-
3. Are raw material samples labeled with lot number, container, the date and the person who collected the sample?原料样品标识是否标有批号,容器编号,日期,以及专门负责收集该样品的人?
-
4. Are COA’s required for raw material approval?原料审批是否需要出厂合格证明?
-
5. Are representative samples of each lot of raw material pulled and tested/examined for physical and/or chemical characteristics as specified?每批原材料是否按照规定和技术指标取样进行物理和/或化学特性测试/检验?
-
6. Are bagged or boxed raw materials stored off the floor?是否有袋装或盒装的原材料直接储存在地板上?
-
7. Is FIFO used for raw materials?原材料是否遵循先进先出原则?
-
8. Is there a quarantine system for rejected raw materials designed to prevent their use?是否有隔离体系确保被拒的原料不会被使用
-
9. Do you use any form of sterilization on raw materials?是否对原料进行灭菌处理?
-
10. Do you recycle/reuse any raw materials in the manufacture of other components?在生产其他包材时,是否回收/重复使用任何原材料?
CALIBRATION
-
11. Is there a procedure established to control, calibrate and maintain inspection, measuring, and test equipment (including testing software) that is used to demonstrate the conformance of product to specified requirements?是否设立程序来控制、校准、检查维护、测量和测试设备(包括测试软件)?这些设备是用来验证产品符合规定的要求。
-
12. Is there an instrument list of all the testing instruments that require calibration?是否有所有需要校准的测试仪器清单?
-
13. Is there documentation of periodic calibration of the testing instruments showing that the instruments were calibrated on schedule to a preset tolerance and with a traceable reference standard?是否有定期校准仪器的文件(校准计划/追溯清单)表明仪器符合预定的公差和可追溯的参考标准下按时校准?
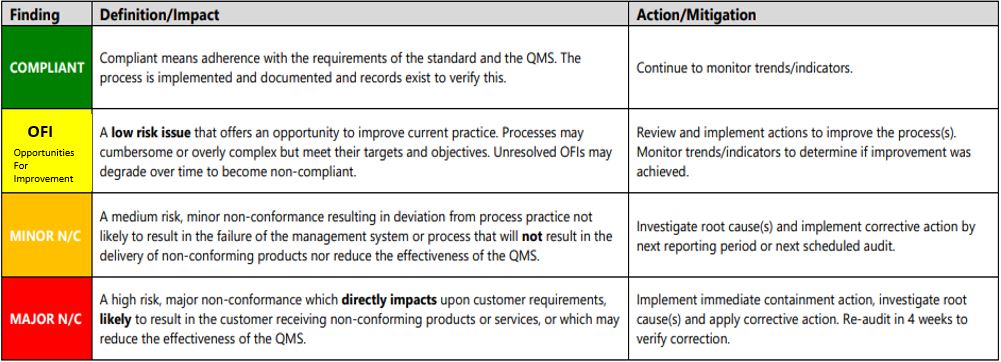
- Compliant
- OFI
- Minor N/C
- Major N/C
- N/A
PROCESSING AREA
-
14. Are there written production and process control procedures approved by the QC unit?是否有书面的生产和过程控制程序经QC部门批准?
-
15. Are there written procedures for the cleaning and sanitation of equipment?是否有流程文件关于设备清洁和消毒程序?
-
16. Is the cleaning/sanitization procedure validated?清洁/卫生程序是否有效?
-
17. Are there cleaning/sanitization records for the equipment?设备是否有清洁/卫生记录?
-
18. Is the equipment cleaning/sanitization status labeled?设备清洁/卫生状态是否有标示?
-
19. Is the equipment inspected for cleanliness before use and documented in the production record?设备在使用前是否进行清洁检查并记录在生产记录中?
-
20. Is the production area free from materials from previous operations (line clearance)?生产区域是否没有以前操作遗留的物料(是否清线)?
-
21. Are there procedures to ensure correct mold/tooling is being used?是否有程序确保使用正确的模具/工具?
-
22. Are all major equipment identified and documented in the batch production record?生产批记录中是否有所有主要设备的标识和文件记录?
-
23. Are key processing steps and parameters documented in the production records?关键工艺步骤和参数是否记录在生产记录中?
-
24. Is the compressed air that comes into component contact oil-free and micro filtered?接触组件的压缩空气是否无油、微过滤?
PRODUCTION
-
25. Are proper drawings and specifications being used in production?在生产中是否使用了正确的图纸和标准?
-
26. Are labels held in quarantine until issued to the production area?标签是否单独存储直到用于生产?
-
27. Are in-process components identified with a label or tag?生产过程中的包材是否有标签进行标识?
-
28. Is finished product examined during finishing to assure correct labeling or decoration?成品在加工过程中是否经过检验以确保被正确标识或装饰?
-
29. Do you have procedures for minimizing out-of-spec components and or out-of-spec labeling/decoration?是否有程序最小化不合格包材和标签的影响?
-
30. Are finished product standards refreshed every 6 months?成品样品是否每6个月更新一次?
-
31. Are production samples retained for at least four years from manufacture?大货样品是否从生产开始至少保留四年?
-
32. Are finished product standards stored in an environmentally controlled area to prevent product contamination and degradation?成品样品是否储存在环境控制区域以防止产品污染和降解?
-
33. Are there procedures to control, use, and reconcile components that are undecorated, decorated, or labeled?是否有程序来控制、使用和协调未加工的、已加工的或被标记的包材?
-
34. Are labels and other proprietary components cleared, stored, and/or destructed post-production?标签和其他专有包材是否在生产后被清除、储存和/或销毁?
HOLDING AND DISTRIBUTION
-
35. Are there written warehousing procedures describing quarantine of components or labels until approved by QC?是否有书面的仓库管理程序以规定包材或标签的分区存储,直到QC批准使用?
-
36. Are rejected finished products status labeled or held in a quarantine area?被拒绝放行的成品是否有状态标签或保存在隔离区?
-
37. Are there written procedures for disposal of rejected finished product in a manner that assures it is not used?是否有书面的程序处理不合格的成品以确保它没有被使用?
-
38. Is there a SOP that describes the requirements for product release, specification compliance, samples and documents?是否有SOP来描述产品放行、标准合规性、样品和文件的要求?
-
39. Are transit testing trials conducted based on transportation methods typically used?运输测试试验是否基于常用的运输方法进行?
-
40. Do you have procedures in place to ensure all components that are undecorated, decorated, and/or labeled are packaged, stored, handled, and transported in a way that will help minimize/prevent damage?是否程序来确保所有未加工的、已加工的和/或贴有标签的包材在包装、储存、加工和运输过程中最小化/防止损坏?
LOSS PREVENTION
-
41. Are there adequate controls to prevent the loss of finished goods and/or componentry from the facility?工厂是否有足够的管控措施来防止成品和/或零部件的遗失?
-
42. Is facility access controlled and monitored after hours? Are the access records reviewed?下班后是否对工厂出入进行管控和监控?是否会审核访问记录?
-
43. Are procedures in place for the proper notification and destruction of unacceptable/obsolete finished goods and/or package components?是否有适当的程序通知和销毁不可接受/废弃的成品和/或包装部件?
-
44. Are products shipped in a controlled & secured manner to prevent tampering, contamination or loss during transit?产品是否以受控和安全的方式运输,以防止在运输过程中被篡改、污染或丢失?
BUILDING AND FACILITIES
-
45. The outside of the building is maintained free of trash and debris that provide harborage.建筑的外部没有垃圾和装修的残骸。
-
46. The building size and construction facilitate cleaning maintenance and operations.建筑物的大小和结构便于清洁、维护和操作。
-
47. There is adequate space to prevent mix-ups of component (ingredient)s, labels, materials, and to prevent contamination.有足够的空间以防止包材(部件)、标签、材料的混淆,并防止污染。
-
48. Doors and windows are kept closed.门窗保持常闭状态。
-
49. Operations are performed within specifically defined areas to prevent contamination or mix-ups.操作必须在特定的区域内进行,以防止污染或混淆。
-
50. Is there adequate lighting?光照是否充足?
-
51. Is there adequate filtered ventilation to production operations where product is exposed?产品暴露的生产操作区域是否有足够的过滤通风?
-
52. There are adequate drains for equipment and to prevent standing water.有足够的排水设备以预防存水。
-
53. The building is free of signs of infestation.建筑物没有虫害的迹象。
-
54. There are written procedures for pest control including a drawing or floor plan showing the placement of control devices.有书面的虫害控制程序,包括显示控制装置放置位置的图纸或平面图。
-
55. Is the building maintained in a good state of repair?建筑物是否处于良好的维修状态?
Record & Documentation Review
PRODUCTION RECORDS
-
56. Is the QC unit review of the records documented?QC部门对文件记录是否审核?
-
57. Does the review result in a shipping release document?审核结果是否体现在出货放行文件里?
-
58. Was all testing completed before the shipping release was signed?所有的测试是否在货物放行单签署前完成?
-
59. For first production has the contract manufacturer established an internal procedure to identify the First Production? Give written notification at least 48 hours before start-up. Assure that before start-up there is an approved standard, approved specification, fill and assembly specification and packaging specification.对于第一次生产,合同制造商是否建立了内部程序来识别第一次生产?至少在启动前48小时书面通知。确保在开始前有一个批准的标准和规范,包括灌装,装配以及包装规范。
-
60. Are production records retained for at least 4 years and/or the time period required by local regulations?生产记录是否保留至少4年和/或当地法规要求的时间期限?
REGISTERED FACILITY
-
61. Has the facility been registered with local governing agencies as a cosmetic, chemical, or similar manufacturing facility and has received approval to manufacture legally within the guidelines as specified by the registration?工厂是否已在当地管理机构注册为化妆品、化学品或类似的生产工厂,并已获得批准在注册规定的范围内合法生产?
-
62. Has the facility been certified, under local governing agencies and/or to a international standard for the manufacturing of cosmetic, chemical or similar products? (Ex. ISO 9001, ISO 22716, etc.)工厂是否通过了当地管理机构和/或化妆品、化学品或类似产品制造的国际标准的认证?(如ISO 9001、ISO 22716等)
-
63. When was the last local government inspection? Have any major non-compliances and/or fines been issued in the past 10 years? 上次当地政府检查是什么时候?在过去10年是否有重大违规及/或罚款?
-
64. Are production records retained for one year past expiration date?生产记录是否在过期后保留一年?
-
65. Are there written procedures for an annual review of written records; batch production records, complaints, recalls and investigations?对于书面记录、批次生产记录、投诉、召回和调查的年度审查是否有书面程序?
-
66. Is there a record of the most recent required review for Mast products? Or an example of a review from a similar customer?是否有Mast产品最近要求的审查的记录?或者一个类似客户的例子?
-
67. Are their established requirements and/or certifications required for all subcontractors and suppliers?所有分包商和供应商是否都需要符合所拟定的要求和/或资质?
QUALITY CONTROL
-
68. Does the facility have a Quality Manual? Is the manual updated and audited through change control?工厂是否有质量手册?更新和审核手册是否通过变更控制程序?
-
69. Does the QC unit have written procedures to approve or reject all component raw materials, containers, closures, in-process materials, packaging material, labeling, and finished products?QC部门是否有书面程序来批准或拒绝所有零部件原材料、外箱、瓶盖、生产中用料、包装材料、标签和成品?
-
70. Are adequate laboratory facilities available to the QC unit for testing and approval or rejection of all component raw materials, in-process materials, packaging material, labeling, and finished product?QC部门是否有足够的实验设施来测试、批准或拒绝所有的原材料,生产中用料,包装材料,标签和成品?
-
71. Does the QC unit approve all procedures and specifications impacting the identity, strength, quality, and purity of the product? QC部门是否批准所有影响产品标识、强度、质量和纯度的程序和规范?
-
72. Does the QC Unit review production records prior to release or shipping the product?QC部门是否在放行或发运产品前审核生产记录?
-
73. Is there history of internal and/or external quality holds, withdrawals or recalls?是否有内部和/或外部质量持有,撤销或召回的历史?
-
74. Is there an appropriate CAPA system in place to identify and resolve quality based issues?是否有适当的CAPA系统来识别和解决质量问题?
-
75. Are finished goods inspected properly by QC prior to release for shipping?成品在发货前是否经QC检验?
-
76. Does the vendor audit their raw material suppliers?供应商是否对原材料供应商进行审核?
PERSONNEL QUALIFICATIONS
-
77. Is there a record of training in GMP and in the particular operations each employee performs?是否有每个员工在GMP和特定操作方面的培训记录?
-
78. Is the training conducted by a qualified individual and is a retraining cadence specified?是否由具有资质的人进行培训,是否规定了再培训的频率?
-
79. Do the personnel use protective apparel such as head, face and arm coverings properly to prevent product contamination?员工是否正确使用防护用具,如头、脸和手臂遮盖物,以防止产品污染?
SIGN-OFF
-
Auditor E-Signature & Date审核员签字&日期: