Title Page
-
Document No.
-
Audit Title
-
Client / Site
-
Conducted on
-
Prepared by
-
Location
-
Personnel
Investigation Report
-
Food safety incident is an incident which has the potential to cause injury, illness or death. These types of incident are likely to affect consumers and customers, disrupt business and may have an adverse impact on GF's reputation.
-
1.0 Site
-
2.0 Incident Number
-
3.0 Source
-
4.0 Date of Incident
-
5.0 Time of Incident
-
6.0 Product Description
-
7.0 Batch Code (s) (Best Before/Use By)
8.0 Overview
-
Nature of Incident
-
Hazard Type
-
Is the Incident Contained ?
-
Are we rejecting product ?
-
Amount of product rejected.
-
Cause of the issue.
-
Root Cause
-
Overall Risk Score
-
Corrective Actions
-
Preventive Actions
-
Description of event
-
Add Photos as required
9.0 Team Members Involved in Investigating the Incident
-
Name and Sign
-
Name and Sign
-
Name and Sign
-
Name and Sign
-
Name and Sign
-
Name and Sign
-
Name and Sign
-
Name and Sign
10.0 Investigation Summary
-
Summary
-
Photos as reqd
11.0 Capture Facts
-
What do you know for certain ?
-
Evidence
-
Photos as reqd
12.0 Consolidate Assumptions
-
What do you believe has occurred or contributed to the situation ?
13.0 Root Cause
-
Detail the System that failed
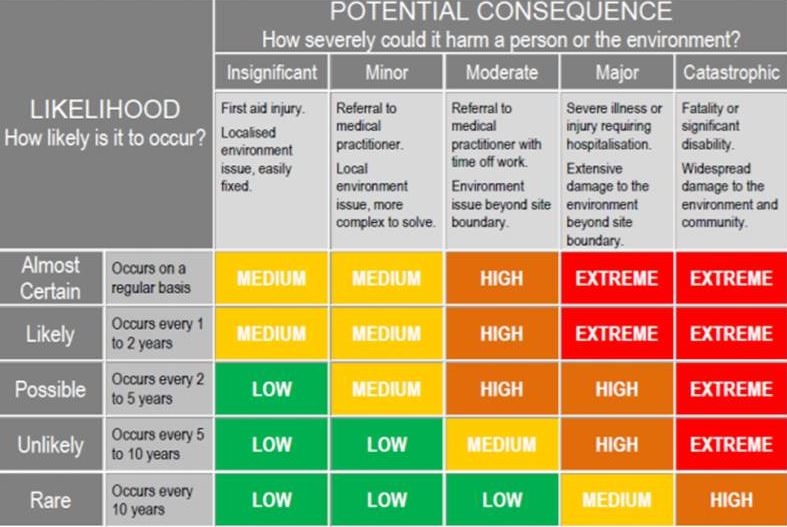
14.0 Potential Risk
-
-
What is the Likelihood ?
-
What is the consequence ?
-
Overall Risk Score - as per risk assessment table.
15.0 Corrective Actions
-
Actions taken immediately to rectify the problem. Include Person Responsible
-
Actions taken immediately to rectify the problem. Include Person Responsible
-
Actions taken immediately to rectify the problem. Include Person Responsible
-
Name and Signature of person responsible to ensure actions are carried out.
16.0 Preventive Actions
-
Medium and Long Term Action required to prevent a repeat incident.
-
Medium and Long Term Action required to prevent a repeat incident.
-
Medium and Long Term Action required to prevent a repeat incident.
-
Name and Signature of person responsible to ensure actions are carried out.
17.0 Report Date
-
Date of Report
18.0 Investigated By:
-
Name and Signature of Lead.
-
NB:
1. Treat with Urgency.
2. Site QA to complete Serious Incident Report within 2 hours of incident and send to designated National QA representitive.
3. National QA representative is to circulate the Serious Incident Inter Company Investigation alert to all GF sites and relevant Senior Executives as appropriate and maintain investigation records. Note: where a Product Withdrawal or Recall is initiated the Group Quality Director will review the alert prior to circulation.
4. Investigation documentation is to be attached to the NCR in the Non-Conformance database.
Serious Incident - Inter Company Investigation
-
Can this happen at your site ?<br>If No , record why this cannot happen at your site.
-
If the same incident can occur on your site then complete the following fields.
-
Investigation details: Record details of the site investigation carried out.
-
Name and signature of person who investigated the incident at your site.
-
Record any action taken to prevent occurrence:
-
Record preventative action to be taken to prevent occurrence.
-
Report date
-
Name and Signature of person who has completed this report.