Title Page
-
Site conducted
-
Snowfox SOP 11-1B: Internal Plant Audit for Manufacturing Facilities
-
Conducted on
-
Inspector
-
Location
-
Each question has to be classified and scored in any of the following categories: Yes (1) - No (0)
A company standard of 90% is considered a passing score. A score below 90% is considered a Fail.
This Internal Plant Audit is based on the Audit Standards Comparison to the FDA Preventive Controls for Human Food Rule template provided by the US Government to comply with the Current Good Manufacturing Practices provisions in 21 CFR part 117 as applicable to Seafood HACCP: https://www.fda.gov/downloads/Food/GuidanceRegulation/FSMA/UCM602404.pdf
Note: As per the 'Seafod HACCP and the FDA Food Safety Modernization Act: Guidance for Industry' document provided by FDA, seafood processors are exempt from the requirements of Current Good Manufacturing Practice and Preventive Controls Regulation subpart C, Hazard Analysis and Risk-Based Preventive Controls, and subpart G, Supply-Chain Program, if the seafood processor is in compliance with the seafood HACCP regulation (part 123). These won't be assessed in this internal plant audit:
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=117&showFR=1&subpartNode=21:2.0.1.1.16.2 -
For non-conformances with immediate corrective actions, describe corrective action/s taken. Person responsible for corrective action/s must sign/initial.
-
For non-conformances requiring work/service orders or maintenance to be scheduled, assign an Action to the Plant Manager with a description, priority level, and a target completion date.
cGMP Section §117 Personnel
-
Instructions:
1. Select “Yes” for compliance and “No” for non-compliance.
2. If “No,” specify the non-conformance under “Notes” and add photos under “Photo” of the non-compliant item.
3. If “No,” select the appropriate corrective action and specify the corrective action/s taken under notes and/or attach photos of the corrective action/s.
For special/unique cases, select “Other Corrective Action/s” and specify in the notes.
§117.10 (a) Disease Control
-
- Anyone suffering from a condition that could contaminate food, food-contact surfaces, or packaging materials is excluded from food handling areas
- Anyone who works in a food handling area and who has a disease or illness, symptoms of a disease or illness or an open or infected lesion is required to report their condition to a responsible person at the establishment -
There are no employees with open or infected wounds, skin infections or sores. Employees with open wounds on exposed areas of the skin are adequately covered with waterproof dressing.
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
There are no employees suffering from a disease or illness that could contaminate food, food-contact surfaces, or packaging materials.
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
§117.10 (b) Cleanliness
-
Requirement: Employees must maintain personal hygiene as per SOP 1
-
Employees wear protective clothing (smock, boots) and hair restraint/s (hair net/beard net) that are clean and suitable for the tasks that they are charged to perform to protect against contamination. Protective clothing are removed immediately upon leaving processing area.
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Employees are washing and sanitizing hands thoroughly: upon entering production area, after each absence from the work station or after each changeover, and at any other time when the hands may have become soiled.
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Employees do not wear jewellery or personal adornments (ie. false eyelashes, false nails, nail polish, topical medicinal creams/hand creams) that could become incorporated into fish being processed.
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Employees change gloves after each changeover and anytime when the gloves may have become soiled, damaged, or contaminated.
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Gloves are to be maintained in an intact, clean and sanitary condition.
-
There are no pills, medications, food and drink items, and personal belongings in food processing areas. There is no smoking, spitting, eating, drinking, chewing gum or using tobacco in process areas.
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
§117.20 Plant and Grounds
§117.20 Plant and Grounds
-
(1) Properly stored equipment and grounds properly maintained.
(2) Maintaining roads, yards, and parking lots so that they do not constitute a source of contamination -
Surrounding land is free of debris, refuse, pooling water, dead vegetation or pest harborages. External waste containers are clean and equipped with tight fitting lids that are resistant to animals/pests
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Waste containers in the facility are identifiable, secured & removed at a set frequency, or more often if necessary
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Waste containers must not be allowed to overflow
-
The facility is designed so that the flow of incoming ingredients, persons, chemicals, packaging materials and equipment does not pose a risk of contamination to the food
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
There should be adequate space for equipment and storage of materials (in between pallets)
-
Floors, walls, ceilings are constructed and maintained so they may be adequately cleaned and in good repair (condensation in control)
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Floors, walls, and ceilings must be: (1) cleanable - no cracks or gaps that may impede cleaning; wall to floor joints must be sealed, (2) in good repair - no rust, peeling paint, leaking lubricants, (3) properly sloped - floors sloped to allow for efficient drainage and (4) free of condensation - no condensate from fixtures, ducts and pipes
-
Placement and intensity of lighting in the establishment is appropriate to effectively produce food, conduct inspection, and allow for cleaning. Light fixtures are shielded with covers to protect against breakage or lights are shatter-proof
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
U.S. FDA Food Code: 6-303.11
108 LUX: 75 cm above floor, walk-in refrigeration units and dry food storage areas
215 LUX: 75 cm above floor in areas used for handwashing, warewashing, and equipment/utensil storage, and in toilet rooms
540 LUX: 75 cm above floor where a food employee is working with food or with utensils or equipment -
Processing Area
-
Kitchen / Rice Cooking Room
-
Staging / Packaging Room
-
Finished Product Cooler
-
Dish Room
-
Freezer
-
Raw Material Cooler
-
Dry Storage / Receiving Area
-
Offices
-
The ventilation system prevents the accumulation of heat, steam, condensation or dust
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Building is constructed and maintained to prevent pest and animal entry.
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Check for gaps or holes in the wall, floor.
Check for gaps underneath main entrances (if you can see gap, this is a potential entry for pests)
§117.35 Sanitary Operations, §117.37 Sanitary Facilities and Controls
-
117.35 (a) General Maintenance
Buildings, fixtures, and other physical facilities must be maintained in a clean and sanitary condition and must be kept in repair adequate to prevent food from becoming adulterated. -
Buildings, fixtures, and overhead coolers are functioning as intended, maintained in a clean and sanitary condition and must be kept in repair adequate to prevent food from becoming adulterated (no rust, peeling paint).
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
117.35 (b) Substances used in cleaning and sanitizing
-
(1) Cleaning compounds and sanitizing agents must be free from undesirable microorganisms and must be safe and adequate under conditions of use.
(2) Toxic cleaning compounds must be identified, held and stored in a manner to protect against contamination -
All chemicals in the Approved Chemical List have a filed Letter of Guarantee from the supplier
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Sanitizing chemicals are not expired and dispensers are at the appropriate chemical concentration for their intended purpose and are food-grade (SOP 2-3).
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Spot-check chemical concentration: Vegetable Wash Disinfectant
-
Dish Sanitizer
-
Floor Sanitizer
-
Sanitizing chemicals are clearly labelled and stored in a designated room or area, away from production, that is dry and well-ventilated. In process areas, spray bottles are kept away from food.
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
There should be so sanitizers and non-food chemical agents near food, food-contact surface, or food-packaging materials. All chemicals in the Approved Chemical List should have a filed Letter of Guarantee from the supplier.
-
Ensure that staff are washing utensils appropriately and as needed (i.e. in between changeovers)
117. 35 (c) Pest Control
-
Pest Control
- Pest must not be allowed in any area of a food plant.
- Effective measures must be taken to exclude pests from the manufacturing, processing, packing and holding areas of food.
- The use of pesticides to control pests in the plant is permitted only under precautions are restrictions that will protect against the contamination of food, food contact surfaces and packaging. -
All pest control devices are operating as intended and have been serviced appropriately as per contract
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Visual verification indicate no evidence of pests, particularly in processing area
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
117.35 (d) Sanitation of food-contact and non-food-contact surfaces
-
All food-contact surfaces must be cleaned as frequently as necessary to protect against allergen cross-contact and against contamination of food.
-
Single-service articles such as paper towels are stored, handled, and disposed of in a manner that prevents cross-contamination
-
All food-contact surfaces, non-food-contact surfaces, equipment, and utensils are cleaned and sanitized before use and after any interruption during which the food-contact surfaces may have become contaminated.
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
i.e., if table was used to hand-roll LTO and the table needs to be used to be used for Shrimp Pad Thai bowls, the table must be sufficiently cleaned prior to next use
117.35 (e) Sanitation of non-food-contact surfaces
-
Sanitation of non-food-contact surfaces of equipment must be cleaned in a manner as necessary to protect against allergen cross-contact and from contamination.
-
Non-food-contact surfaces of equipment are cleaned as frequently as needed and away from food preparation
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Single-service articles such as paper towels are stored, handled, and disposed of in a manner that prevents cross-contamination
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
117.35 (f) Storage and handling of cleanable portable equipment and utensils
-
Storage and handling of cleanable portable equipment and utensils
Cleaned and sanitized portable equipment with food-contact surfaces and utensils must be stored in a location and manner that protects food-contact surfaces from allergen cross-contact and from contamination. -
Cleaned portable equipment and utensils such as knives are stored separately from those that are soiled and in a manner that protects from allergen contamination and cross-contamination
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
§117.37 Sanitary Facilities and Controls
-
§117.37 (a) (b) Water supply and Plumbing
The water supply and plumbing must be adequate for the operations intended and must be derived from an adequate source and be sanitary if it comes into contact with food of food-contact surfaces.
Note: No ice/steam is used in the facility
U.S. FDA Food Code Requirements:
Hand-washing = 40°C +/- 2°C (100 to 108°F) [US FDA Food Code 5-202.12]
Warewashing = 71°C (160°F) as per US FDA Food Code 4-302.13 -
Water is supplied in adequate quantity, temperature, and pressure for processing, cleaning, hand-washing, etc.
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Check that the temperatures can reach the appropriate temperatures
-
117.37 (c) Sewage Disposal
Sewage must be disposed of into an adequate sewage system -
Sewage is disposed of by third-party contractor every 3 months or as needed.
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Ensure that service was done as scheduled and filed.
§117.37 (d) Employee Facilities
-
Each plant must provide employees with adequate, readily accessible toilet facilities.
Each plant must provide hand-washing facilities designed to ensure that an employee's hands are not a source of contamination by providing facilities that are adequate, convenient, and furnish running water at a suitable temperature.
Note: 117.37 (f) Rubbish and offal disposal is not applicable to this factory -
Change rooms have sufficient storage for street clothing that is separate from storage for protective clothing to be worn during food production
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
There are sufficient hand washing stations, lavatories, showers, drinking water stations, lunch rooms, and change rooms are properly located, designed and maintained
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Hand washing stations are equipped with warm water, non-hand operated taps, liquid soap, non-hand operated paper towel dispenser, and garbage bin.
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Hand-washing notices are posted in lavatories and at the entrances to food production areas.
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Washrooms are located separate from production, maintained in good operating order and equipped with toilet paper
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
§117.40 Equipment and Utensils
117.40 (a) (b) (c) Equipment and Utensils
-
All plant equipment and utensils used in processing, packing, or holding food must be designed to be adequately cleanable, and must be maintained to protect against allergen cross-contact and contamination
Note: 117.40 (d) is not applicable -
All equipment, utensils, containers, and controlling/measuring devices are used only for the premises' intended purpose and functioning as intended.
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
All equipment, utensils, containers, and controlling/measuring devices are cleanable and in good repair (no rust, damage, peeling paint, leaking lubricants)
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Check that there are no crevices on equipment as they will no longer be cleanable
-
All equipment, utensils, containers, and controlling/measuring devices are maintained in a clean and sanitary condition during changeovers.
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
All equipment, utensils, containers, and controlling/measuring devices in-use are calibrated.
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Check that all scales, thermometers, and pH meters in-use at the facility are properly calibrated. Check that metal detectors, ink date coder and labeller are not due for service.
117.40 (e) Temperature and Humidity
-
Temperature and humidity are maintained at levels appropriate for the food.
-
Each freezer and cold storage compartment used to store and hold food capable of supporting growth of microorganisms must be fitted with an indicating thermometer, temperature measuring device, or temperature recording device so installed as to show the temperature accurately within the compartment
-
Note: 117.80(g) is not applicable to this facility
-
Production areas are maintained at a temperature that prevents the growth of bacteria [ ≤10°C (50°F) ]
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Freezers maintain fish at ≤ -18°C (-0.4°F)
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Coolers maintain fish at ≤ 4°C (39.2°F)
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Refrigerated areas are equipped with a recording thermometer
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Refrigeration facilities operated in a manner that prevents frost build-up
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
§117.80 Processes and Controls
-
117.80 (a) (b) (c) Competencies and qualifications
Appropriate quality control must be employed to ensure that food is suitable for human consumption: -
Employees have the necessary accreditations or certifications necessary for their duties
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Check Employee files if their training records are up-to-date (GMPs, Allergens) and also check if the following are updated:
- Food Handler's Certificate
- Food Defense 101
Monitoring critical control points (CCPs): CCP 1
-
The pH should be between 3.8 to 4.2. If pH > 4.2, add more vinegar. If pH < 3.8, add more rice and re-check. If this cannot be achieved, product should be discarded. Reference: SOP 4 Regulations
-
Batch/Bin#
-
Rice pH
Rice Temperature
-
Spot-check of rice pH matches pH recorded by rice cooker and verifier
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
Monitoring critical control points (CCPs): CCP 2
-
Note: Check all packs removed for thawing. Attach photos under "Photo"
-
Spot-check of frozen seafood thawed overnight indicate that a cut has been made on the package.
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
Monitoring critical control points (CCPs): CCP 3
-
-
Spot-check of finished products indicate that the finished products have the correct labels applied (i.e., Label: California Deluxe Sushi Roll = Contents: California Deluxe Sushi Roll).
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Spot-check of finished products indicate that the list of ingredients are correct.
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Spot-check of finished products indicate that the list of allergens are correct.
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
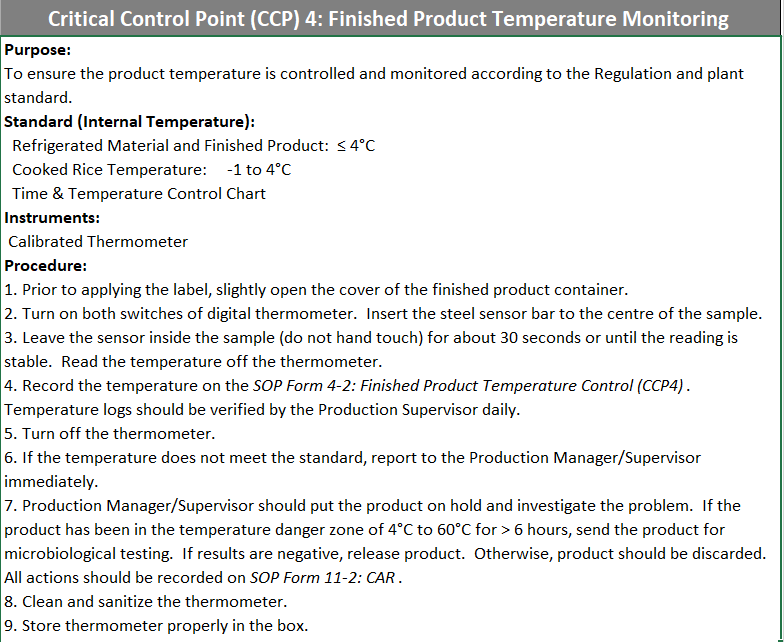
Monitoring critical control points (CCPs): CCP 4
-
-
Production Date and Time
Cooler Temperature
-
Spot-check of a finished product temperature indicated that CCP 4 was met (temperature is ≤ 40F)
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Specify the finished product name under "Notes"
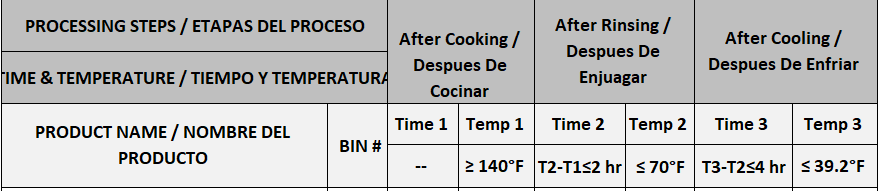
Monitoring critical control points (CCPs): CCP 5
-
Reference: SOP 4 Regulations
-
Batch/Bin#
-
Time 1
Temperature 1
-
Time 2
Temperature 2
-
Time 3
Temperature 3
-
Spot-check indicates rice/noodle temperature meets CCP 5
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
In-Process and Finished Product Weight Control
-
In-process Weight
-
Finished Product Weight
-
Random weight check of sushi matches weight recorded
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Note in-process and finished product item under "Notes"
Inspection of measures to protect against inclusion of metal or extraneous materials in food - Blade inspection (SOP 4-5c)
-
All blades used in production are in good condition (i.e., no chipped blade, no rust)
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
Receiving Process
-
Received items are moved to appropriate cold storages within 30 minutes; Correct lot codes recorded on SOP 5-1
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
Vegetable Washing Procedure
-
Vegetables are washed and treated according to SOP 4
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Check that vegetables are washed with AFVT for at least 90 seconds and if required, soak for minimum time as per SOP
§117.93 Warehousing and distribution
-
Storage and transportation of food must be under conditions that will protect against allergen cross-contact and against biological (including radiological), chemical and physical contamination.
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Ingredients are stored in their appropriate designated storage location as per allergen alert signages.
-
Finished products are shipped in a pre-cooled conveyance (SOP 4 and SOP 5)
-
Forklifts and pump trucks are maintained in a good operating condition
-
Food are placed on pallets off the floor or on shelves and no item is to be touching the wall
-
Receiving is done only in the designated Receiving Area and food is received separately from non-food chemical agents
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Receiving is controlled during unloading/loading to prevent entry of pests or other hazards by ensuring that the loading doors are opened for a short duration as possible.
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
"First-In, First-Out" stock rotation principle is followed. There are no food stored that has passed its expiration date.
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
-
Unused equipment are stored in designated locations that are clean and away from production area
-
Corrective Action/s
- Removed
- Washed, Rinsed, and Sanitized
- Re-trained Personnel
- Maintenance / Work Order Scheduled
Verification
-
Inspector
-
Verified by